The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury
- PMID: 36551285
- PMCID: PMC9775635
- DOI: 10.3390/biom12121856
The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury
Abstract
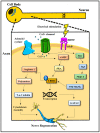
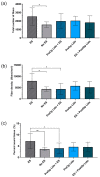
Peripheral nerve injuries (PNI) are common and often result in lifelong disability. The peripheral nervous system has an inherent ability to regenerate following injury, yet complete functional recovery is rare. Despite advances in the diagnosis and repair of PNIs, many patients suffer from chronic pain, and sensory and motor dysfunction. One promising surgical adjunct is the application of intraoperative electrical stimulation (ES) to peripheral nerves. ES acts through second messenger cyclic AMP to augment the intrinsic molecular pathways of regeneration. Decades of animal studies have demonstrated that 20 Hz ES delivered post-surgically accelerates axonal outgrowth and end organ reinnervation. This work has been translated clinically in a series of randomized clinical trials, which suggest that ES can be used as an efficacious therapy to improve patient outcomes following PNIs. The aim of this review is to discuss the cellular physiology and the limitations of regeneration after peripheral nerve injuries. The proposed mechanisms of ES protocols and how they facilitate nerve regeneration depending on timing of administration are outlined. Finally, future directions of research that may provide new perspectives on the optimal delivery of ES following PNI are discussed.
Keywords: electrical stimulation; nerve regeneration; nerve repair; peripheral nerve.
Conflict of interest statement
A.M. Moore has a Research Collaboration with Checkpoint Surgical. No other conflict of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Electrical stimulation to enhance peripheral nerve regeneration: Update in molecular investigations and clinical translation.Exp Neurol. 2020 Oct;332:113397. doi: 10.1016/j.expneurol.2020.113397. Epub 2020 Jul 3. Exp Neurol. 2020. PMID: 32628968 Review.
-
Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats.Exp Neurol. 2015 Jul;269:142-53. doi: 10.1016/j.expneurol.2015.03.022. Epub 2015 Apr 2. Exp Neurol. 2015. PMID: 25842267
-
Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans.Neurotherapeutics. 2016 Apr;13(2):295-310. doi: 10.1007/s13311-015-0415-1. Neurotherapeutics. 2016. PMID: 26754579 Free PMC article. Review.
-
Promoting Nerve Regeneration: Electrical Stimulation, Gene Therapy, and Beyond.Physiology (Bethesda). 2022 Nov 1;37(6):0. doi: 10.1152/physiol.00008.2022. Epub 2022 Jul 12. Physiology (Bethesda). 2022. PMID: 35820181 Review.
-
Brief Electrical Stimulation Accelerates Axon Regeneration and Promotes Recovery Following Nerve Transection and Repair in Mice.J Bone Joint Surg Am. 2021 Oct 20;103(20):e80. doi: 10.2106/JBJS.20.01965. J Bone Joint Surg Am. 2021. PMID: 34668879
Cited by
-
Brief Electrical Stimulation Promotes Recovery after Surgical Repair of Injured Peripheral Nerves.Int J Mol Sci. 2024 Jan 4;25(1):665. doi: 10.3390/ijms25010665. Int J Mol Sci. 2024. PMID: 38203836 Free PMC article. Review.
-
Effect of Metformin on the Functional and Electrophysiological Recovery of Crush Injury-Induced Facial Nerve Paralysis in Diabetic Rats.J Pers Med. 2023 Aug 27;13(9):1317. doi: 10.3390/jpm13091317. J Pers Med. 2023. PMID: 37763084 Free PMC article.
-
Treatment of Denervated Muscle Atrophy by Injectable Dual-Responsive Hydrogels Loaded with Extracellular Vesicles.Adv Sci (Weinh). 2025 Mar;12(10):e2412248. doi: 10.1002/advs.202412248. Epub 2025 Jan 21. Adv Sci (Weinh). 2025. PMID: 39836492 Free PMC article.
-
The Dynamics of Nerve Degeneration and Regeneration in a Healthy Milieu and in Diabetes.Int J Mol Sci. 2023 Oct 16;24(20):15241. doi: 10.3390/ijms242015241. Int J Mol Sci. 2023. PMID: 37894921 Free PMC article. Review.
-
Research progress in different physical therapies for treating peripheral nerve injuries.Front Neurol. 2025 Apr 7;16:1508604. doi: 10.3389/fneur.2025.1508604. eCollection 2025. Front Neurol. 2025. PMID: 40260135 Free PMC article. Review.
References
-
- Javeed S., Faraji A., Dy C., Ray W., MacEwan M. Application of electrical stimulation for peripheral nerve regeneration: Stimulation parameters and future horizons. Interdiscip. Neurosurg. 2021;24:101117. doi: 10.1016/j.inat.2021.101117. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

