Concomitant Administration of Red Ginseng Extract with Lactic Acid Bacteria Increases the Plasma Concentration of Deglycosylated Ginsenosides in Healthy Human Subjects
- PMID: 36551324
- PMCID: PMC9775652
- DOI: 10.3390/biom12121896
Concomitant Administration of Red Ginseng Extract with Lactic Acid Bacteria Increases the Plasma Concentration of Deglycosylated Ginsenosides in Healthy Human Subjects
Abstract
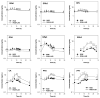
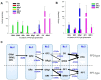
With the increased frequency of red ginseng extract (RGE) and lactic acid bacteria (LAB) co-administration, we aimed to investigate the interactions between RGE and LAB with regard to in vitro and in vivo deglycosylation metabolism and the pharmacokinetics of ginsenosides. As a proof-of-concept study, five healthy humans were administered RGE (104.1 mg of total ginsenosides/day) with or without co-administration of LAB (2 g, 1 billion CFU/day) for 2 weeks, and the plasma concentrations of ginsenosides in human plasma were monitored. The plasma exposure to compound K (CK), ginsenoside Rh2 (GRh2), protopanaxadiol (PPD), and protopanaxatriol (PPT) in the concomitant administration RGE and LAB groups increased by 2.7-, 2.1-, 1.6-, and 3.5-fold, respectively, compared to those in the RGE administration group, without a significant change in Tmax. The plasma concentrations of GRb1, GRb2, and GRc remained unchanged, whereas the AUC values of GRd and GRg3 significantly decreased in the concomitant administration RGE and LAB groups. To understand the underlying mechanism, the in vitro metabolic activity of ginsenosides was measured during the fermentation of RGE or individual ginsenosides in the presence of LAB for 1 week. Consistent with the in vivo results, co-incubation with RGE and LAB significantly increased the formation rate of GRh2, CK, PPD, and PPT. These results may be attributed to the facilitated deglycosylation of GRd and GRg3 and the increased production of GRh2, CK, PPD, and PPT by the co-administration of LAB and RGE. In conclusion, LAB supplementation increased the plasma concentrations of deglycosylated ginsenosides, such as GRh2, CK, PPD, and PPT, through facilitated deglycosylation metabolism of ginsenosides in the intestine.
Keywords: deglycosylation metabolism; ginsenosides; lactic acid bacteria (LAB); pharmacokinetics; red ginseng extract (RGE).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Effect of Lactic Acid Bacteria on the Pharmacokinetics and Metabolism of Ginsenosides in Mice.Pharmaceutics. 2021 Sep 17;13(9):1496. doi: 10.3390/pharmaceutics13091496. Pharmaceutics. 2021. PMID: 34575573 Free PMC article.
-
Pharmacokinetics of ginsenosides following repeated oral administration of red ginseng extract significantly differ between species of experimental animals.Arch Pharm Res. 2020 Dec;43(12):1335-1346. doi: 10.1007/s12272-020-01289-0. Epub 2020 Nov 22. Arch Pharm Res. 2020. PMID: 33225388
-
Detection of 13 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, Compound K, 20(S)-Protopanaxadiol, and 20(S)-Protopanaxatriol) in Human Plasma and Application of the Analytical Method to Human Pharmacokinetic Studies Following Two Week-Repeated Administration of Red Ginseng Extract.Molecules. 2019 Jul 18;24(14):2618. doi: 10.3390/molecules24142618. Molecules. 2019. PMID: 31323835 Free PMC article.
-
Ginsenosides are active ingredients in Panax ginseng with immunomodulatory properties from cellular to organismal levels.J Ginseng Res. 2022 Nov;46(6):711-721. doi: 10.1016/j.jgr.2021.12.007. Epub 2021 Dec 22. J Ginseng Res. 2022. PMID: 36312737 Free PMC article. Review.
-
[Research achievements on ginsenosides biosynthesis from Panax ginseng].Zhongguo Zhong Yao Za Zhi. 2016 Dec;41(23):4292-4302. doi: 10.4268/cjcmm20162302. Zhongguo Zhong Yao Za Zhi. 2016. PMID: 28933103 Review. Chinese.
Cited by
-
Pharmacokinetics and Enterohepatic Circulation of 2-(Quinoline-8-carboxamido)benzoic Acid (2-QBA) in Mice.Pharmaceutics. 2024 Jul 12;16(7):934. doi: 10.3390/pharmaceutics16070934. Pharmaceutics. 2024. PMID: 39065631 Free PMC article.
-
Compound K-enriched Korean red ginseng prevents lung cancer progression by targeting cancer cells and fibroblasts.J Ginseng Res. 2025 Jul;49(4):438-450. doi: 10.1016/j.jgr.2025.03.010. Epub 2025 Apr 4. J Ginseng Res. 2025. PMID: 40621073 Free PMC article.
-
Application of probiotic bacteria in ginsenoside bioconversion and enhancing its health-promoting benefits: a review.Food Sci Biotechnol. 2024 Nov 12;34(8):1631-1659. doi: 10.1007/s10068-024-01734-6. eCollection 2025 Apr. Food Sci Biotechnol. 2024. PMID: 40160953 Review.
-
Metabolism and drug interactions of Korean ginseng based on the pharmacokinetic properties of ginsenosides: Current status and future perspectives.J Ginseng Res. 2024 May;48(3):253-265. doi: 10.1016/j.jgr.2024.02.003. Epub 2024 Mar 4. J Ginseng Res. 2024. PMID: 38707645 Free PMC article. Review.
-
In Vitro Metabolism and Transport Characteristics of Zastaprazan.Pharmaceutics. 2024 Jun 13;16(6):799. doi: 10.3390/pharmaceutics16060799. Pharmaceutics. 2024. PMID: 38931920 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

