Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review
- PMID: 36551407
- PMCID: PMC9774632
- DOI: 10.3390/antibiotics11121750
Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review
Abstract
Heterocyclic compounds are considered as one of the major and most diverse family of organic compounds. Nowadays, the demand for these compounds is increasing day-by-day due to their enormous synthetic and biological applications. These heterocyclic compounds have unique antibacterial activity against various Gram-positive and Gram-negative bacterial strains. This review covers the antibacterial activity of different heterocyclic compounds with nitrogen moiety. Some of the derivatives of these compounds show excellent antibacterial activity, while others show reasonable activity against bacterial strains. This review paper aims to bring and discuss the detailed information on the antibacterial activity of various nitrogen-based heterocyclic compounds. It will be helpful for the future evolution of diseases to synthesize new and effective drug molecules.
Keywords: heterocyclic compound; in-vitro studies; infectious disease; medicinal chemistry; organic synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

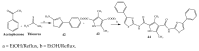















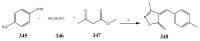

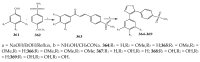


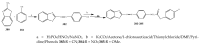

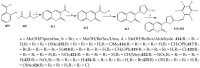



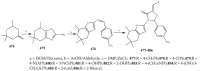

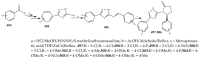


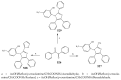
References
-
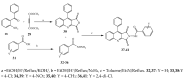
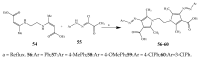
- Cheddie A., Shintre S.A., Bantho A., Mocktar C., Koorbanally N.A. Synthesis and antibacterial activity of a series of 2-trifluoromethylbenzimidazole-thiazolidinone derivatives. J. Heterocycl. Chem. 2020;57:299–307. doi: 10.1002/jhet.3777. - DOI
-
- Sreedevi R., Saranya S., Anilkumar G. Recent Trends in the Silver-Catalyzed Synthesis of Nitrogen Heterocycles. Adv. Synth. Catal. 2019;361:4625–4644. doi: 10.1002/adsc.201900599. - DOI
-
- Ghaemi M., Pordel M. Isoxazolo [4, 3-e] indazole as a new heterocyclic system: Design, synthesis, spectroscopic characterization, and antibacterial activity. Chem. Heterocycl. Compd. 2016;52:52–57. doi: 10.1007/s10593-016-1833-7. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

