New Insights into the Mechanism of Antibacterial Action of Synthetic Peptide Mo-CBP3-PepI against Klebsiella pneumoniae
- PMID: 36551410
- PMCID: PMC9774128
- DOI: 10.3390/antibiotics11121753
New Insights into the Mechanism of Antibacterial Action of Synthetic Peptide Mo-CBP3-PepI against Klebsiella pneumoniae
Abstract
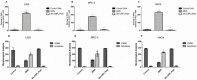
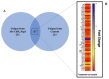
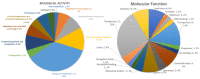
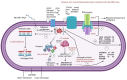
Klebsiella pneumoniae is a multidrug-resistant opportunistic human pathogen related to various infections. As such, synthetic peptides have emerged as potential alternative molecules. Mo-CBP3-PepI has presented great activity against K. pneumoniae by presenting an MIC50 at a very low concentration (31.25 µg mL-1). Here, fluorescence microscopy and proteomic analysis revealed the alteration in cell membrane permeability, ROS overproduction, and protein profile of K. pneumoniae cells treated with Mo-CBP3-PepI. Mo-CBP3-PepI led to ROS overaccumulation and membrane pore formation in K. pneumoniae cells. Furthermore, the proteomic analysis highlighted changes in essential metabolic pathways. For example, after treatment of K. pneumoniae cells with Mo-CBP3-PepI, a reduction in the abundance of protein related to DNA and protein metabolism, cytoskeleton and cell wall organization, redox metabolism, regulation factors, ribosomal proteins, and resistance to antibiotics was seen. The reduction in proteins involved in vital processes for cell life, such as DNA repair, cell wall turnover, and protein turnover, results in the accumulation of ROS, driving the cell to death. Our findings indicated that Mo-CBP3-PepI might have mechanisms of action against K. pneumoniae cells, mitigating the development of resistance and thus being a potent molecule to be employed in producing new drugs against K. pneumoniae infections.
Keywords: antibacterial peptides; multidrug-resistant bacteria; proteomic analysis; synthetic peptides.
Conflict of interest statement
The authors report no conflict of interest. The authors alone are responsible for the content and the writing of the paper.
Figures



Similar articles
-
No Chance to Survive: Mo-CBP3-PepII Synthetic Peptide Acts on Cryptococcus neoformans by Multiple Mechanisms of Action.Antibiotics (Basel). 2023 Feb 12;12(2):378. doi: 10.3390/antibiotics12020378. Antibiotics (Basel). 2023. PMID: 36830289 Free PMC article.
-
United we stand, divided we fall: in-depth proteomic evaluation of the synergistic effect of Mo-CBP3-PepI and Ciprofloxacin against Staphylococcus aureus biofilms.Biofouling. 2023 Aug-Sep;39(8):838-852. doi: 10.1080/08927014.2023.2279992. Epub 2023 Nov 20. Biofouling. 2023. PMID: 37955278
-
Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII are synthetic antimicrobial peptides active against human pathogens by stimulating ROS generation and increasing plasma membrane permeability.Biochimie. 2019 Feb;157:10-21. doi: 10.1016/j.biochi.2018.10.016. Epub 2018 Oct 31. Biochimie. 2019. PMID: 30389515
-
Computational approach, scanning electron and fluorescence microscopies revealed insights into the action mechanisms of anticandidal peptide Mo-CBP3-PepIII.Life Sci. 2021 Sep 15;281:119775. doi: 10.1016/j.lfs.2021.119775. Epub 2021 Jun 26. Life Sci. 2021. PMID: 34186044
-
Innate Host Defense against Klebsiella pneumoniae and the Outlook for Development of Immunotherapies.J Innate Immun. 2022;14(3):167-181. doi: 10.1159/000518679. Epub 2021 Oct 8. J Innate Immun. 2022. PMID: 34628410 Free PMC article. Review.
Cited by
-
No Chance to Survive: Mo-CBP3-PepII Synthetic Peptide Acts on Cryptococcus neoformans by Multiple Mechanisms of Action.Antibiotics (Basel). 2023 Feb 12;12(2):378. doi: 10.3390/antibiotics12020378. Antibiotics (Basel). 2023. PMID: 36830289 Free PMC article.
-
Behind the Curtain: In Silico and In Vitro Experiments Brought to Light New Insights into the Anticryptococcal Action of Synthetic Peptides.Antibiotics (Basel). 2023 Jan 11;12(1):153. doi: 10.3390/antibiotics12010153. Antibiotics (Basel). 2023. PMID: 36671354 Free PMC article.
-
Nicotianin-I: A Tobacco Floral Nectar Peptide with Anticandidal Activity.ACS Omega. 2025 May 14;10(20):20213-20225. doi: 10.1021/acsomega.4c10806. eCollection 2025 May 27. ACS Omega. 2025. PMID: 40454052 Free PMC article.
References
-
- O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Government of the United Kingdom; London, UK: 2016.
-
- About Antibiotic Resistance|CDC. [(accessed on 11 November 2022)]; Available online: https://www.cdc.gov/drugresistance/about.html.
-
- Klebsiella Pneumoniae in Healthcare Settings|HAI|CDC. [(accessed on 11 November 2022)]; Available online: https://www.cdc.gov/hai/organisms/klebsiella/klebsiella.html.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

