Analysis of the Oral Microbiome in a Patient with Cardiofaciocutaneous Syndrome and Severe Periodontal Disease: Impact of Systemic Antibiotic Therapy
- PMID: 36551411
- PMCID: PMC9774349
- DOI: 10.3390/antibiotics11121754
Analysis of the Oral Microbiome in a Patient with Cardiofaciocutaneous Syndrome and Severe Periodontal Disease: Impact of Systemic Antibiotic Therapy
Abstract
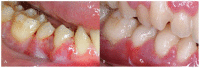
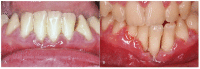
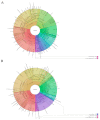
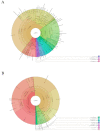
An 8-year-old girl diagnosed with cardiofaciocutaneous syndrome presented to our department with gingival pain, inflammation, and bleeding. Her medical history included hypoplasia of the corpus callosum, intellectual disability, trichothiodystrophy, global developmental delay, myopia, laryngomalacia, hypothyroidism, and osteoporosis. A diagnosis was reached of "periodontitis as a direct manifestation of systemic diseases". During 9 years of follow-up, there were exacerbation episodes with spontaneous gum bleeding, ulcers in the interdental papilla, tooth mobility, and progressive tooth loss. Some of these exacerbation episodes resolved clinically with the administration of amoxicillin and metronidazole. We therefore proposed an oral microbiome study (subgingival and saliva samples) before and after antibiotic therapy. The most abundant genera at the subgingival level before administering antibiotics were Prevotella, Streptococcus, Fusobacterium, Leptotrichia, and Aggregatibacter. Of the 94 genera sequenced, 57 were less abundant in the post-treatment state than at baseline, particularly certain Gram-negative periodontal pathogens such as Porphyromonas, Treponema, Aggregatibacter, Fusobacterium, and Campylobacter. In contrast, other genera related to oral health, such as Haemophilus, Granulicatella, and Abiotrophia, showed an increase after administering the antibiotic. In conclusion, periodontitis exacerbations as a direct manifestation of systemic disease can occasionally be controlled exclusively with systemic antibiotics, without the need for performing mechanical periodontal therapy. This clinical recovery is correlated to substantial changes in the oral microbiome, which lead to the recovery of eubiosis of the microbiota.
Keywords: antibiotics; cardiofaciocutaneous syndrome; microbiome; periodontitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Periodontitis associated with plasminogen deficiency: a case report.BMC Oral Health. 2015 May 14;15:59. doi: 10.1186/s12903-015-0045-3. BMC Oral Health. 2015. PMID: 25971786 Free PMC article.
-
Change in subgingival microbial profiles in adult periodontitis subjects receiving either systemically-administered amoxicillin or metronidazole.J Clin Periodontol. 2001 Jul;28(7):597-609. doi: 10.1034/j.1600-051x.2001.028007597.x. J Clin Periodontol. 2001. PMID: 11422580
-
Pyrosequencing Analysis of Subgingival Microbiota in Distinct Periodontal Conditions.J Dent Res. 2015 Jul;94(7):921-7. doi: 10.1177/0022034515583531. Epub 2015 Apr 22. J Dent Res. 2015. PMID: 25904141
-
Effect of adjunctive systemic antibiotics on microbial populations compared with scaling and root planing alone for the treatment of periodontitis: A pilot randomized clinical trial.J Periodontol. 2022 Apr;93(4):570-583. doi: 10.1002/JPER.20-0764. Epub 2021 Sep 14. J Periodontol. 2022. PMID: 34374434 Clinical Trial.
-
ASSOCIATION BETWEEN PERIODONTITIS AND LIVER DISEASE.Acta Clin Croat. 2022 Feb;60(3):510-518. doi: 10.20471/acc.2021.60.03.22. Acta Clin Croat. 2022. PMID: 35282488 Free PMC article. Review.
Cited by
-
Cross-cultural translation and modification of the revised oral assessment guide for oral health assessment by non-dentists.BDJ Open. 2023 Sep 12;9(1):42. doi: 10.1038/s41405-023-00168-2. BDJ Open. 2023. PMID: 37699888 Free PMC article.
-
Spotlight on therapeutic efficiency of green synthesis metals and their oxide nanoparticles in periodontitis.J Nanobiotechnology. 2024 Jan 5;22(1):21. doi: 10.1186/s12951-023-02284-5. J Nanobiotechnology. 2024. PMID: 38183090 Free PMC article. Review.
-
Case Report: Shift from Aggressive Periodontitis to Feline Chronic Gingivostomatitis Is Linked to Increased Microbial Diversity.Pathogens. 2025 Feb 26;14(3):228. doi: 10.3390/pathogens14030228. Pathogens. 2025. PMID: 40137713 Free PMC article.
-
Recent advances in metal nanoparticles to treat periodontitis.J Nanobiotechnology. 2023 Aug 21;21(1):283. doi: 10.1186/s12951-023-02042-7. J Nanobiotechnology. 2023. PMID: 37605182 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

