Variability of Mycobacterium avium Complex Isolates Drug Susceptibility Testing by Broth Microdilution
- PMID: 36551413
- PMCID: PMC9774755
- DOI: 10.3390/antibiotics11121756
Variability of Mycobacterium avium Complex Isolates Drug Susceptibility Testing by Broth Microdilution
Abstract
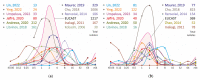
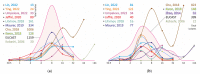
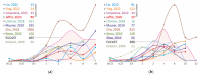
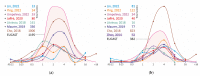
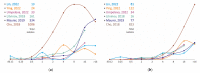
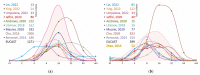
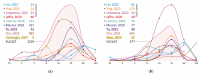
Non-tuberculous mycobacteria are widely distributed in environments and are capable of infecting humans, particularly those with a compromised immune system. The most prevalent species that cause nontuberculous mycobacterial lung diseases are slow-growing bacteria from the Mycobacterium avium complex (MAC), mainly M. avium or M. intracellulare. The key treatment of MAC infections includes macrolides, ethambutol, and rifampicin; however, the therapy outcomes are unsatisfactory. Phenotypic drug susceptibility testing is a conditional recommendation prior to treatment, and critical concentrations for clarithromycin, amikacin, moxifloxacin, and linezolid have been established. In this review, data from studies on the determination of MIC of clinical isolates using the broth microdilution method were summarized. A significant variation in the MIC distributions from different studies was found. The main reasons could impact the findings: insufficient reproducibility of the phenotypic testing and variation in species lineages identified in different laboratories, which could have various intrinsic susceptibility to drugs. For most of the drugs analyzed, the MICs are too high, which could undermine the treatment efficiency. Further improvement of treatment outcomes demands the validation of microbiological resistance criteria together with the identification of molecular mechanisms of resistance.
Keywords: MAC complex; MIC; amikacin; avium; ethambutol; intracellular; macrolides; nontuberculous mycobacteria; resistance.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Towards clinical breakpoints for non-tuberculous mycobacteria - Determination of epidemiological cut off values for the Mycobacterium avium complex and Mycobacterium abscessus using broth microdilution.Clin Microbiol Infect. 2023 Jun;29(6):758-764. doi: 10.1016/j.cmi.2023.02.007. Epub 2023 Feb 20. Clin Microbiol Infect. 2023. PMID: 36813087
-
Antimicrobial susceptibility and minimum inhibitory concentration distribution of common clinically relevant non-tuberculous mycobacterial isolates from the respiratory tract.Ann Med. 2022 Dec;54(1):2500-2510. doi: 10.1080/07853890.2022.2121984. Ann Med. 2022. PMID: 36120867 Free PMC article.
-
Differences in drug susceptibility pattern between Mycobacterium avium and Mycobacterium intracellulare isolated in respiratory specimens.J Infect Chemother. 2018 Apr;24(4):315-318. doi: 10.1016/j.jiac.2017.10.022. Epub 2017 Dec 7. J Infect Chemother. 2018. PMID: 29223615
-
Drug susceptibility testing of nontuberculous mycobacteria.Future Microbiol. 2014;9(9):1095-110. doi: 10.2217/fmb.14.60. Future Microbiol. 2014. PMID: 25340838 Review.
-
Treatment strategies with alternative treatment options for patients with Mycobacterium avium complex pulmonary disease.Respir Investig. 2022 Sep;60(5):613-624. doi: 10.1016/j.resinv.2022.05.006. Epub 2022 Jun 30. Respir Investig. 2022. PMID: 35781424 Review.
Cited by
-
The Drug Susceptibility of Non-Tuberculous Mycobacteria (NTM) in a Referral Hospital in Rome from 2018 to 2023.Microorganisms. 2024 Aug 8;12(8):1615. doi: 10.3390/microorganisms12081615. Microorganisms. 2024. PMID: 39203457 Free PMC article.
-
Susceptibility Patterns in Clinical Isolates of Mycobacterium avium Complex from a Hospital in Southern Spain.Microorganisms. 2024 Dec 17;12(12):2613. doi: 10.3390/microorganisms12122613. Microorganisms. 2024. PMID: 39770815 Free PMC article.
-
Implications of Intravenous and Inhaled Amikacin Breakpoint Reporting for Mycobacterium avium Complex Pulmonary Isolates.Pathogens. 2025 Jun 12;14(6):583. doi: 10.3390/pathogens14060583. Pathogens. 2025. PMID: 40559591 Free PMC article.
References
-
- Chaptal M., Andrejak C., Bonifay T., Beillard E., Guillot G., Guyomard-Rabenirina S., Demar M., Trombert-Paolantoni S., Jacomo V., Mosnier E., et al. Epidemiology of Infection by Pulmonary Non-Tuberculous Mycobacteria in French Guiana 2008-2018. PLoS Negl. Trop. Dis. 2022;16:e0010693. doi: 10.1371/journal.pntd.0010693. - DOI - PMC - PubMed
-
- Sun Q., Yan J., Liao X., Wang C., Wang C., Jiang G., Dong L., Wang F., Huang H., Wang G., et al. Trends and Species Diversity of Non-Tuberculous Mycobacteria Isolated From Respiratory Samples in Northern China, 2014–2021. Front. Public Health. 2022;10:923968. doi: 10.3389/fpubh.2022.923968. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

