Resistance to Critical Important Antibacterials in Staphylococcus pseudintermedius Strains of Veterinary Origin
- PMID: 36551415
- PMCID: PMC9774309
- DOI: 10.3390/antibiotics11121758
Resistance to Critical Important Antibacterials in Staphylococcus pseudintermedius Strains of Veterinary Origin
Abstract
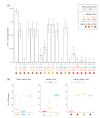
Staphylococcal infections represent a challenge in companion animals and hospitalized patients. This study aimed to assess the resistance of Staphylococcus pseudintermedius isolates, against a broad panel of antibacterials, including exclusive to human medicine. A total of 40 S. pseudintermedius were collected from clinical specimens of dogs (n = 31) and cats (n = 5). All strains were tested for 20 antibacterials, namely 14 Critical Important and eight Highly Important Antibacterials (CIA and HIA, respectively), indicative for 18 antimicrobial classes. All strains were susceptible to seven antibiotics (daptomycin, fosfomycin, fusidic acid, linezolid, quinupristin-dalfopristin, teicoplanin/vancomycin, tigecycline). The highest resistance was against penicillin (97.5% Confidence Interval [CI]: 83.8-100.0), whereas the lowest against telavancin (2.5%, CI: 0.0-16.2). Resistance versus Highest Priority CIA was observed, namely against macrolides (70.0, CI: 52.1-84.3), quinolones (62.5, CI: 44.5-78.3), 5th generation cephalosporins (7.5, CI: 1.3-21.6), and glycopeptides (2.5%, CI: 0.0-14.2). Among High Priority CIA, strains were resistant only to aminoglycosides (65.0, CI: 47.0-80.4) and ansamycins (12.5, CI: 3.8-28.1). We observed the highest resistance against veterinary medicine antibacterials, but there was also resistance against antibacterials exclusive to human medicine, namely ceftaroline (7.5, CI: 1.0-23.8) and telavancin. S. pseudintermedius zoonotic potential and its rate of acquisition of new resistance should encourage surveillance on a broad spectrum of antibacterials.
Keywords: Italy; Staphylococcus pseudintermedius; antimicrobial resistance; antimicrobial susceptibility testing; cat; critically important antimicrobials; dog.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus.J Antimicrob Chemother. 2006 Aug;58(2):338-43. doi: 10.1093/jac/dkl235. Epub 2006 Jun 20. J Antimicrob Chemother. 2006. PMID: 16787952
-
Antimicrobial susceptibility of Staphylococcus pseudintermedius colonizing healthy dogs in Saskatoon, Canada.Can Vet J. 2016 Jan;57(1):65-9. Can Vet J. 2016. PMID: 26740701 Free PMC article.
-
[Investigation of antibiotic resistance patterns and reduced vancomycin susceptibilities of methicillin-resistant Staphylococcus aureus isolates: a multi-center study].Mikrobiyol Bul. 2015 Apr;49(2):240-8. doi: 10.5578/mb.9230. Mikrobiyol Bul. 2015. PMID: 26167824 Turkish.
-
New antibiotics for the treatment of severe staphylococcal infection in the critically ill patient.Curr Opin Crit Care. 2005 Oct;11(5):481-6. doi: 10.1097/01.ccx.0000176690.18433.22. Curr Opin Crit Care. 2005. PMID: 16175036 Review.
-
Antimicrobial resistance in methicillin susceptible and methicillin resistant Staphylococcus pseudintermedius of canine origin: literature review from 1980 to 2013.Vet Microbiol. 2014 Jul 16;171(3-4):337-41. doi: 10.1016/j.vetmic.2014.02.008. Epub 2014 Feb 18. Vet Microbiol. 2014. PMID: 24613081 Review.
Cited by
-
In vitro activity of selected antimicrobials against methicillin-resistant Staphylococcus pseudintermedius of canine origin in Poland.Vet Med Sci. 2024 May;10(3):e1385. doi: 10.1002/vms3.1385. Vet Med Sci. 2024. PMID: 38547160 Free PMC article.
-
Antimicrobial Resistance Characterization of Methicillin-Resistant Staphylococcus aureus and Staphylococcus pseudintermedius Isolates from Clinical Cases in Dogs and Cats in Belgium.Antibiotics (Basel). 2025 Jun 20;14(7):631. doi: 10.3390/antibiotics14070631. Antibiotics (Basel). 2025. PMID: 40723934 Free PMC article.
-
Emergence of novel methicillin resistant Staphylococcus pseudintermedius lineages revealed by whole genome sequencing of isolates from companion animals and humans in Scotland.PLoS One. 2024 Jul 5;19(7):e0305211. doi: 10.1371/journal.pone.0305211. eCollection 2024. PLoS One. 2024. PMID: 38968222 Free PMC article.
-
Multidrug-Resistant Commensal and Infection-Causing Staphylococcus spp. Isolated from Companion Animals in the Valencia Region.Vet Sci. 2024 Jan 26;11(2):54. doi: 10.3390/vetsci11020054. Vet Sci. 2024. PMID: 38393072 Free PMC article.
-
Zoonoses in dog and cat shelters in North-East Italy: update on emerging, neglected and known zoonotic agents.Front Vet Sci. 2024 Nov 27;11:1490649. doi: 10.3389/fvets.2024.1490649. eCollection 2024. Front Vet Sci. 2024. PMID: 39664895 Free PMC article.
References
-
- Devriese L.A., Vancanneyt M., Baele M., Vaneechoutte M., De Graef E., Snauwaert C., Cleenwerck I., Dawyndt P., Swings J., Decostere A., et al. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int. J. Syst. Evol. Microbiol. 2005;55:1569–1573. doi: 10.1099/ijs.0.63413-0. - DOI - PubMed
-
- PubMed Staphylococcus pseudintermedius—Search Results. [(accessed on 21 October 2022)]; Available online: https://pubmed.ncbi.nlm.nih.gov/?term=Staphylococcus+pseudintermedius&so....
-
- O’Neill A.M., Worthing K.A., Kulkarni N., Li F., Nakatsuji T., McGrosso D., Mills R.H., Kalla G., Cheng J.Y., Norris J.M., et al. Antimicrobials from a feline commensal bacterium inhibit skin infection by drug-resistant S. pseudintermedius. eLife. 2021;10:e66793. doi: 10.7554/eLife.66793. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

