In Vitro Study on Green Propolis as a Potential Ingredient of Oral Health Care Products
- PMID: 36551420
- PMCID: PMC9774696
- DOI: 10.3390/antibiotics11121764
In Vitro Study on Green Propolis as a Potential Ingredient of Oral Health Care Products
Abstract
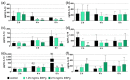
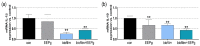
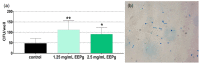
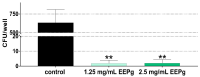
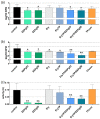
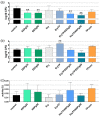
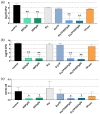
Propolis is increasingly being discussed as an alternative to commonly used antiseptics. This in vitro study focused on the ethanolic extract of green Brazilian propolis (EEPg) as an additive in an oral health care product. We investigated (i) a potential inflammation-modulation activity of EEPg when a periodontal or Candida biofilm was exposed to monocytic (MONO-MAC-6) cells, (ii) the adhesion of oral pathogens to gingival keratinocytes and (iii) the antimicrobial and antibiofilm effect of different toothpaste formulations. EEPg decreased the levels of interleukin (IL)-1β and increased IL-10 in MONO-MAC cells challenged with a periodontal biofilm. In contact with TIGK cells, EEPg reduced the numbers of adherent Porphyromonas gingivalis to 0.5% but did not affect the adhesion of Candida albicans. The frequent brushing of a cariogenic biofilm with a toothpaste supplemented with EEPg reduced the surface microhardness loss of enamel specimens. Mixing an experimental erythritol toothpaste with 25 and 50 mg/mL of EEPg confirmed the antibacterial activity of EEPg against oral bacteria and particularly inhibited periodontal biofilm formation. The suggested toothpaste formulations seem to have potential in the prevention of caries, gingivitis and periodontitis and should be evaluated in further in vitro research and in clinical trials.
Keywords: Brazilian green propolis; biofilm; monocytic cells; toothpaste.
Conflict of interest statement
A.L. is consultant at EMS, Nyon, Switzerland. The other authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Efficacy of toothpaste containing Brazilian green propolis extracts with an optimal kaempferide/betuletol ratio for improving oral microbiota: A randomized, controlled, paired crossover study.J Ethnopharmacol. 2025 Jan 30;337(Pt 1):118762. doi: 10.1016/j.jep.2024.118762. Epub 2024 Aug 29. J Ethnopharmacol. 2025. PMID: 39214193 Clinical Trial.
-
In Vitro Activity of Propolis on Oral Microorganisms and Biofilms.Antibiotics (Basel). 2021 Aug 26;10(9):1045. doi: 10.3390/antibiotics10091045. Antibiotics (Basel). 2021. PMID: 34572627 Free PMC article.
-
Activity of antifungal drugs and Brazilian red and green propolis extracted with different methodologies against oral isolates of Candida spp.BMC Complement Med Ther. 2021 Nov 24;21(1):286. doi: 10.1186/s12906-021-03445-5. BMC Complement Med Ther. 2021. PMID: 34814913 Free PMC article.
-
Propolis, Aloe Vera, Green Tea, Cranberry, Calendula, Myrrha and Salvia Properties against Periodontal Microorganisms.Microorganisms. 2022 Oct 31;10(11):2172. doi: 10.3390/microorganisms10112172. Microorganisms. 2022. PMID: 36363764 Free PMC article. Review.
-
Efficacy of triclosan-based toothpastes in the prevention and treatment of plaque-induced periodontal and peri-implant diseases.Minerva Stomatol. 2013 Mar;62(3):71-88. Minerva Stomatol. 2013. PMID: 23518778 Review. English, Italian.
Cited by
-
Herbal remedies for oral and dental health: a comprehensive review of their multifaceted mechanisms including antimicrobial, anti-inflammatory, and antioxidant pathways.Inflammopharmacology. 2025 Mar;33(3):1085-1160. doi: 10.1007/s10787-024-01631-8. Epub 2025 Feb 5. Inflammopharmacology. 2025. PMID: 39907951 Free PMC article. Review.
-
In-Vitro Effect of Manuka Honey / Propolis Toothpastes on Bacteria and Biofilm Associated with Caries and Gingivitis.Oral Health Prev Dent. 2025 Mar 27;23:203-210. doi: 10.3290/j.ohpd.c_1910. Oral Health Prev Dent. 2025. PMID: 40146163 Free PMC article.
-
Complexification of In Vitro Models of Intestinal Barriers, A True Challenge for a More Accurate Alternative Approach.Int J Mol Sci. 2023 Feb 10;24(4):3595. doi: 10.3390/ijms24043595. Int J Mol Sci. 2023. PMID: 36835003 Free PMC article. Review.
References
-
- González-Serrano J., López-Pintor R.M., Serrano J., Torres J., Hernández G., Sanz M. Short-term efficacy of a gel containing propolis extract, nanovitamin C and nanovitamin E on peri-implant mucositis: A double-blind, randomized, clinical trial. J. Periodontal Res. 2021;56:897–906. doi: 10.1111/jre.12886. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

