Covalent DNA Binding Is Essential for Gram-Negative Antibacterial Activity of Broad Spectrum Pyrrolobenzodiazepines
- PMID: 36551427
- PMCID: PMC9774941
- DOI: 10.3390/antibiotics11121770
Covalent DNA Binding Is Essential for Gram-Negative Antibacterial Activity of Broad Spectrum Pyrrolobenzodiazepines
Abstract
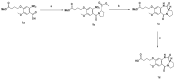
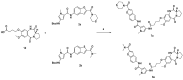
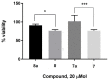
It is urgent to find new antibiotic classes against multidrug-resistant bacteria as the rate of discovery of new classes of antibiotics has been very slow in the last 50 years. Recently, pyrrolobenzodiazepines (PBDs) with a C8-linked aliphatic-heterocycle have been identified as a new broad-spectrum antibiotic class with activity against Gram-negative bacteria. The active imine moiety of the reported lead pyrrolobenzodiazepine compounds was replaced with amide to obtain the non-DNA binding and noncytotoxic dilactam analogues to understand the structure-activity relationship further and improve the safety potential of this class. The synthesised compounds were tested against panels of multidrug-resistant Gram-positive and Gram-negative bacteria, including WHO priority pathogens. Minimum inhibitory concentrations for the dilactam analogues ranged from 4 to 32 mg/L for MDR Gram-positive bacteria, compared to 0.03 to 2 mg/L for the corresponding imine analogues. At the same time, they were found to be inactive against MDR Gram-negative bacteria, with a MIC > 32 mg/L, compared to a MIC of 0.5 to 32 mg/L for imine analogues. A molecular modelling study suggests that the lack of imine functionality also affects the interaction of PBDs with DNA gyrase. This study suggests that the presence of N10-C11 imine moiety is crucial for the broad-spectrum activity of pyrrolobenzodiazepines.
Keywords: antibacterial drug discovery; antimicrobial resistance; broad-spectrum antibiotics; gram-negative bacteria; pyrrolobenzodiazepines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
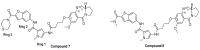

Similar articles
-
New Broad-Spectrum Antibiotics Containing a Pyrrolobenzodiazepine Ring with Activity against Multidrug-Resistant Gram-Negative Bacteria.J Med Chem. 2020 Jul 9;63(13):6941-6958. doi: 10.1021/acs.jmedchem.0c00328. Epub 2020 Jun 25. J Med Chem. 2020. PMID: 32515951
-
C8-Linked Pyrrolobenzodiazepine Monomers with Inverted Building Blocks Show Selective Activity against Multidrug Resistant Gram-Positive Bacteria.ACS Infect Dis. 2018 Feb 9;4(2):158-174. doi: 10.1021/acsinfecdis.7b00130. Epub 2018 Jan 9. ACS Infect Dis. 2018. PMID: 29260545
-
Convergent evolution of antibiotic resistance mechanisms between pyrrolobenzodiazepines and albicidin in multidrug resistant Klebsiella pneumoniae.NPJ Antimicrob Resist. 2025 Jun 6;3(1):52. doi: 10.1038/s44259-025-00104-4. NPJ Antimicrob Resist. 2025. PMID: 40481275 Free PMC article.
-
Facilitating Compound Entry as a Means to Discover Antibiotics for Gram-Negative Bacteria.Acc Chem Res. 2021 Mar 16;54(6):1322-1333. doi: 10.1021/acs.accounts.0c00895. Epub 2021 Feb 26. Acc Chem Res. 2021. PMID: 33635073 Free PMC article. Review.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
Cited by
-
Discovery of new dibenzodiazepine derivatives as antibacterials against intracellular bacteria.RSC Med Chem. 2023 Dec 11;15(1):283-292. doi: 10.1039/d3md00418j. eCollection 2024 Jan 25. RSC Med Chem. 2023. PMID: 38283231 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

