Antimicrobial and Antibiofilm Effect of 4,4'-Dihydroxy-azobenzene against Clinically Resistant Staphylococci
- PMID: 36551456
- PMCID: PMC9774766
- DOI: 10.3390/antibiotics11121800
Antimicrobial and Antibiofilm Effect of 4,4'-Dihydroxy-azobenzene against Clinically Resistant Staphylococci
Abstract
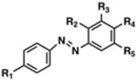
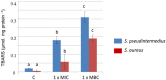
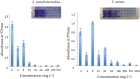
The spread of antibiotic resistance among human and animal pathogens is one of the more significant public health concerns. Moreover, the restrictions on the use of particular antibiotics can limit the options for the treatment of infections in veterinary clinical practice. In this context, searching for alternative antimicrobial substances is crucial nowadays. In this study, 4,4'-dihydroxy-azobenzene (DHAB) was tested for its potential in vitro as an antimicrobial agent against two relevant human and animal pathogens, namely Staphylococcus aureus and Staphylococcus pseudintermedius. The values of minimal inhibitory concentration (MIC) were 64 and 32 mg/L respectively, and they comparable to other azo compounds of probed antimicrobial activity. In addition, the minimal bactericidal concentrations (MCB) were 256 and 64 mg/L. The mechanism by which DHAB produces toxicity in staphylococci has been investigated. DHAB caused membrane damage as revealed by the increase in thiobarbituric acid reactive substances (TBARS) such as malondialdehyde. Furthermore, differential induction of the enzymes peroxidases and superoxide dismutase in S. aureus and S. pseudintermedius suggested their prevalent role in ROS-scavenging due to the oxidative burst induced by this compound in either species. In addition, this substance was able to inhibit the formation of biofilms by both bacteria as observed by colorimetric tests and scanning electron microscopy. In order to assess the relevance of DHAB against clinical strains of MRSA, 10 clinical isolates resistant to either methicillin or daptomycin were assayed; 80% of them gave values of CMI and CMB similar to those of the control S. aureus strain. Finally, cutaneous plasters containing a composite formed by an agar base supplemented with DHAB were designed. These plasters were able to inhibit in vitro the growth of S. aureus and S. pseudintermedius, particularly the later, and this suggests that this substance could be a promising candidate as an alternative to antibiotics in the treatment of animal skin infections, as it has been proven that the toxicity of this substance is very low particularly at a dermal level.
Keywords: Staphylococcus aureus; Staphylococcus pseudintermedius; azo compounds; biofilms; clinical samples; cutaneous plasters; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interests in relation to the present research.
Figures




Similar articles
-
Synergistic Effect of Abietic Acid with Oxacillin against Methicillin-Resistant Staphylococcus pseudintermedius.Antibiotics (Basel). 2021 Jan 15;10(1):80. doi: 10.3390/antibiotics10010080. Antibiotics (Basel). 2021. PMID: 33467635 Free PMC article.
-
Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress.Appl Microbiol Biotechnol. 2020 Oct;104(19):8279-8297. doi: 10.1007/s00253-020-10853-z. Epub 2020 Aug 28. Appl Microbiol Biotechnol. 2020. PMID: 32857200
-
The in vitro antibacterial activity of the anthelmintic drug oxyclozanide against common small animal bacterial pathogens.Vet Dermatol. 2019 Aug;30(4):314-e87. doi: 10.1111/vde.12755. Epub 2019 May 7. Vet Dermatol. 2019. PMID: 31062461
-
In vitro activity of meropenem/piperacillin/tazobactam triple combination therapy against clinical isolates of Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus pseudintermedius and vancomycin-resistant Enterococcus spp.Int J Antimicrob Agents. 2020 Feb;55(2):105864. doi: 10.1016/j.ijantimicag.2019.105864. Epub 2019 Dec 20. Int J Antimicrob Agents. 2020. PMID: 31870598
-
Antimicrobial use of reactive oxygen therapy: current insights.Infect Drug Resist. 2018 Apr 24;11:567-576. doi: 10.2147/IDR.S142397. eCollection 2018. Infect Drug Resist. 2018. PMID: 29731645 Free PMC article. Review.
Cited by
-
Azobenzene as Multi-Targeted Scaffold in Medicinal Chemistry.Molecules. 2024 Dec 12;29(24):5872. doi: 10.3390/molecules29245872. Molecules. 2024. PMID: 39769961 Free PMC article. Review.
-
Unraveling the Formation of Ternary AgCuSe Crystalline Nanophases and Their Potential as Antibacterial Agents.Chem Mater. 2024 Oct 9;36(20):10154-10166. doi: 10.1021/acs.chemmater.4c01604. eCollection 2024 Oct 22. Chem Mater. 2024. PMID: 39464291 Free PMC article.
-
Unveiling the structural aspects of novel azo-dyes with promising anti-virulence activity against MRSA: a deep dive into the spectroscopy via integrated experimental and computational approaches.RSC Adv. 2025 Jan 20;15(3):1665-1679. doi: 10.1039/d4ra06367h. eCollection 2025 Jan 16. RSC Adv. 2025. PMID: 39835211 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

