Strategies to Mitigate and Treat Orthopaedic Device-Associated Infections
- PMID: 36551479
- PMCID: PMC9774155
- DOI: 10.3390/antibiotics11121822
Strategies to Mitigate and Treat Orthopaedic Device-Associated Infections
Abstract
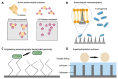
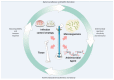
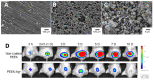
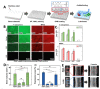
Orthopaedic device implants play a crucial role in restoring functionality to patients suffering from debilitating musculoskeletal diseases or to those who have experienced traumatic injury. However, the surgical implantation of these devices carries a risk of infection, which represents a significant burden for patients and healthcare providers. This review delineates the pathogenesis of orthopaedic implant infections and the challenges that arise due to biofilm formation and the implications for treatment. It focuses on research advancements in the development of next-generation orthopaedic medical devices to mitigate against implant-related infections. Key considerations impacting the development of devices, which must often perform multiple biological and mechanical roles, are delineated. We review technologies designed to exert spatial and temporal control over antimicrobial presentation and the use of antimicrobial surfaces with intrinsic antibacterial activity. A range of measures to control bio-interfacial interactions including approaches that modify implant surface chemistry or topography to reduce the capacity of bacteria to colonise the surface, form biofilms and cause infections at the device interface and surrounding tissues are also reviewed.
Keywords: antimicrobial; biofilm; bioinspired; drug delivery; implant coating; infection; medical device; nanotechnology; orthopaedic implants; polymer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Surface treatment strategies to combat implant-related infection from the beginning.J Orthop Translat. 2018 Sep 28;17:42-54. doi: 10.1016/j.jot.2018.09.001. eCollection 2019 Apr. J Orthop Translat. 2018. PMID: 31194031 Free PMC article. Review.
-
Affinity interactions drive post-implantation drug filling, even in the presence of bacterial biofilm.Acta Biomater. 2017 Jul 15;57:95-102. doi: 10.1016/j.actbio.2017.04.015. Epub 2017 Apr 13. Acta Biomater. 2017. PMID: 28414173 Free PMC article.
-
Impact of multiscale surface topography characteristics on Candida albicans biofilm formation: From cell repellence to fungicidal activity.Acta Biomater. 2024 Mar 15;177:20-36. doi: 10.1016/j.actbio.2024.02.006. Epub 2024 Feb 9. Acta Biomater. 2024. PMID: 38342192 Review.
-
Antimicrobial peptides (AMP) in biofilm induced orthopaedic device-related infections.J Clin Orthop Trauma. 2022 Jan 26;25:101780. doi: 10.1016/j.jcot.2022.101780. eCollection 2022 Feb. J Clin Orthop Trauma. 2022. PMID: 35145849 Free PMC article.
-
Osteointegration, antimicrobial and antibiofilm activity of orthopaedic titanium surfaces coated with silver and strontium-doped hydroxyapatite using a novel blasting process.Drug Deliv Transl Res. 2021 Apr;11(2):702-716. doi: 10.1007/s13346-021-00946-1. Epub 2021 Mar 13. Drug Deliv Transl Res. 2021. PMID: 33713316
Cited by
-
Biofilm tolerance, resistance and infections increasing threat of public health.Microb Cell. 2023 Sep 26;10(11):233-247. doi: 10.15698/mic2023.11.807. eCollection 2023 Nov 6. Microb Cell. 2023. PMID: 37933277 Free PMC article. Review.
-
Enhancing Surgical Outcomes: A Critical Review of Antibiotic Prophylaxis in Orthopedic Surgery.Cureus. 2023 Oct 27;15(10):e47828. doi: 10.7759/cureus.47828. eCollection 2023 Oct. Cureus. 2023. PMID: 38022210 Free PMC article. Review.
-
Surgical Antimicrobial Prophylaxis in Orthopedic Implant Surgeries: An Analysis of Practices, Outcomes, and Costs.Indian J Orthop. 2024 Dec 11;59(2):198-207. doi: 10.1007/s43465-024-01303-3. eCollection 2025 Feb. Indian J Orthop. 2024. PMID: 39886274
-
Biomaterial-Based Scaffolds as Carriers of Topical Antimicrobials for Bone Infection Prophylaxis.Pol J Microbiol. 2025 Jun 18;74(2):232-243. doi: 10.33073/pjm-2025-019. eCollection 2025 Jun 1. Pol J Microbiol. 2025. PMID: 40544521 Free PMC article. Review.
-
Development of a Galleria mellonella Infection Model to Evaluate the Efficacy of Antibiotic-Loaded Polymethyl Methacrylate (PMMA) Bone Cement.Antibiotics (Basel). 2024 Jul 25;13(8):692. doi: 10.3390/antibiotics13080692. Antibiotics (Basel). 2024. PMID: 39199992 Free PMC article.
References
-
- McMillan D.J., Lutton C., Rosenzweig N., Sriprakash K.S., Goss B., Stemberger M., Schuetz M.A., Steck R. Prevention of staphylococcus aures biofilm formation on metallic surgical implants via controlled release of gentamicin. J. Biomed. Sci. Eng. 2011;4:535–542. doi: 10.4236/jbise.2011.48069. - DOI
-
- Evans N.T., Torstrick F.B., Lee C.S.D., Dupont K.M., Safranski D.L., Chang W.A., Macedo A.E., Lin A.S.P., Boothby J.M., Whittingslow D.C., et al. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015;13:159–167. doi: 10.1016/j.actbio.2014.11.030. - DOI - PMC - PubMed
-
- O’ Sullivan C., Kennedy G., O’ Neill L., Crean A.M., Ryan K.B. Biomedical Applications of Inorganic Materials. The Royal Society of Chemistry; Cambridge, UK: 2022. Chapter 5 Inorganic Biomaterials to Support the Formation and Repair of Bone Tissue; pp. 242–304.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

