Staphylococcus aureus- Cure-Associated Antigens Elicit Type 3 Immune Memory T Cells
- PMID: 36551488
- PMCID: PMC9774748
- DOI: 10.3390/antibiotics11121831
Staphylococcus aureus- Cure-Associated Antigens Elicit Type 3 Immune Memory T Cells
Abstract
Background: Staphylococcus aureus is one of the most frequently major mastitis pathogens that cause clinical and subclinical mastitis worldwide. Current antimicrobial treatments are usually ineffective, and the commercially available vaccines lack proven effectiveness. The immunological response elicited by the recombinant S. aureus-cure-associated proteins phosphoglycerate kinase (PGK), enolase (ENO), and elongation factor-G (EF-G) in combination with the granulocyte-macrophage colony-stimulating factor (GM-CSF) DNA vaccination was studied in this work.
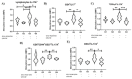
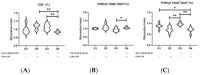
Methods: Here, twenty-three C57BL/6 mice were divided into four groups and vaccinated with: G1: none (control); G2: GM-CSF DNA plasmid DNA vaccine; G3: the combination of EF-G+ENO+PGK; and G4: the combinations of EF-G+ENO+PGK proteins plus GM-CSF plasmid DNA vaccine. After 44 days, spleen cells were collected for immunophenotyping and lymphocyte proliferation evaluation by flow cytometry upon S. aureus stimulus.
Results: Immunization with the three S. aureus recombinant proteins alone resulted in a higher percentage of IL-17A+ cells among CD8+ T central memory cells, as well as the highest intensity of IL-17A production by overall lymphocytes indicating that the contribution of the combined lymphocyte populations is crucial to sustaining a type 3 cell immunity environment.
Conclusion: The immunization with three S. aureus-cure-associated recombinant proteins triggered type 3 immunity, which is a highly interesting path to pursue an effective bovine S. aureus mastitis vaccine.
Keywords: IL-17A; Staphylococcus aureus; T cell response; dairy cow; intramammary infection; mastitis; recombinant antigens; vaccine.
Conflict of interest statement
The authors have a patent titled “Vaccine composition, kit for diagnosis of
Figures


Similar articles
-
Staphylococcus aureus Protection-Related Type 3 Cell-Mediated Immune Response Elicited by Recombinant Proteins and GM-CSF DNA Vaccine.Vaccines (Basel). 2021 Aug 13;9(8):899. doi: 10.3390/vaccines9080899. Vaccines (Basel). 2021. PMID: 34452024 Free PMC article.
-
Memory CD4+ and CD8+ T lymphocyte proliferation in vaccinated dairy cows with different histories of Staphylococcus aureus mastitis.Vet Immunol Immunopathol. 2022 Nov;253:110508. doi: 10.1016/j.vetimm.2022.110508. Epub 2022 Oct 28. Vet Immunol Immunopathol. 2022. PMID: 36327943
-
Immune and experimental infection responses of dairy cows vaccinated with the combination of six Staphylococcus aureus proteins that are expressed during bovine intramammary infection and a triple adjuvant.Vet Immunol Immunopathol. 2021 Aug;238:110290. doi: 10.1016/j.vetimm.2021.110290. Epub 2021 Jun 25. Vet Immunol Immunopathol. 2021. PMID: 34217108
-
Symposium review: Features of Staphylococcus aureus mastitis pathogenesis that guide vaccine development strategies.J Dairy Sci. 2019 May;102(5):4727-4740. doi: 10.3168/jds.2018-15272. Epub 2018 Dec 20. J Dairy Sci. 2019. PMID: 30580940 Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
References
-
- Santos R.P., Souza F.N., Oliveira A.C.D., de Souza Filho A.F., Aizawa J., Moreno L.Z., da Cunha A.F., Cortez A., Della Libera A.M.M.P., Heinemann M.B., et al. Molecular typing and antimicrobial susceptibility profile of Staphylococcus aureus isolates recovered from bovine mastitis and nasal samples. Animals. 2020;10:2143. doi: 10.3390/ani10112143. - DOI - PMC - PubMed
-
- Richardson E.J., Bacigalupe R., Harrison E.M., Weinert L.A., Lycett S., Vrieling M., Robb K., Hoskisson P.A., Holden M.T.G., Feil E.J., et al. Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat. Ecol. Evol. 2018;2:1468–1478. doi: 10.1038/s41559-018-0617-0. - DOI - PMC - PubMed
-
- Campos B., Pickering A.C., Rocha L.S., Aguilar A.P., Fabres-Klein M.H., de Oliveira Mendes T.A., Fitzgerald J.R., de Oliveira Barros Ribon A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022;18:115. doi: 10.1186/s12917-022-03197-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

