Optimization of Pyrazole Compounds as Antibiotic Adjuvants Active against Colistin- and Carbapenem-Resistant Acinetobacter baumannii
- PMID: 36551489
- PMCID: PMC9774939
- DOI: 10.3390/antibiotics11121832
Optimization of Pyrazole Compounds as Antibiotic Adjuvants Active against Colistin- and Carbapenem-Resistant Acinetobacter baumannii
Abstract
The diffusion of antibiotic-resistant, Gram-negative, opportunistic pathogens, an increasingly important global public health issue, causes a significant socioeconomic burden. Acinetobacter baumannii isolates, despite causing a lower number of infections than Enterobacterales, often show multidrug-resistant phenotypes. Carbapenem resistance is also rather common, prompting the WHO to include carbapenem-resistant A. baumannii as a "critical priority" for the discovery and development of new antibacterial agents. In a previous work, we identified several series of compounds showing either direct-acting or synergistic activity against relevant Gram-negative species, including A. baumannii. Among these, two pyrazole compounds, despite being devoid of any direct-acting activity, showed remarkable synergistic activity in the presence of a subinhibitory concentration of colistin on K. pneumoniae and A. baumannii and served as a starting point for the synthesis of new analogues. In this work, a new series of 47 pyrazole compounds was synthesized. Some compounds showed significant direct-acting antibacterial activity on Gram-positive organisms. Furthermore, an evaluation of their activity as potential antibiotic adjuvants allowed for the identification of two highly active compounds on MDR Acinetobacter baumannii, including colistin-resistant isolates. This work confirms the interest in pyrazole amides as a starting point for the optimization of synergistic antibacterial compounds active on antibiotic-resistant, Gram-negative pathogens.
Keywords: Acinetobacter baumannii; ESKAPE bacteria; antibacterials; antibiotic adjuvant; antibiotic potentiation; colistin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
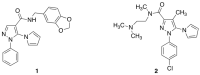




Similar articles
-
PYED-1 Overcomes Colistin Resistance in Acinetobacter baumannii.Pathogens. 2023 Nov 7;12(11):1323. doi: 10.3390/pathogens12111323. Pathogens. 2023. PMID: 38003788 Free PMC article.
-
Screen of Unfocused Libraries Identified Compounds with Direct or Synergistic Antibacterial Activity.ACS Med Chem Lett. 2020 Mar 6;11(5):899-905. doi: 10.1021/acsmedchemlett.9b00674. eCollection 2020 May 14. ACS Med Chem Lett. 2020. PMID: 32435403 Free PMC article.
-
Synergistic Activity of Colistin in Combination with Clofoctol against Colistin Resistant Gram-Negative Pathogens.Microbiol Spectr. 2023 Feb 21;11(2):e0427522. doi: 10.1128/spectrum.04275-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36802038 Free PMC article.
-
Antibiotic-Resistant ESKAPE Pathogens and COVID-19: The Pandemic beyond the Pandemic.Viruses. 2023 Aug 30;15(9):1843. doi: 10.3390/v15091843. Viruses. 2023. PMID: 37766250 Free PMC article. Review.
-
Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria.Antibiotics (Basel). 2023 Nov 3;12(11):1590. doi: 10.3390/antibiotics12111590. Antibiotics (Basel). 2023. PMID: 37998792 Free PMC article. Review.
Cited by
-
4-Trifluoromethyl bithiazoles as broad-spectrum antimicrobial agents for virus-related bacterial infections or co-infections.RSC Med Chem. 2024 Mar 12;15(5):1589-1600. doi: 10.1039/d3md00686g. eCollection 2024 May 22. RSC Med Chem. 2024. PMID: 38784463 Free PMC article.
-
PYED-1 Overcomes Colistin Resistance in Acinetobacter baumannii.Pathogens. 2023 Nov 7;12(11):1323. doi: 10.3390/pathogens12111323. Pathogens. 2023. PMID: 38003788 Free PMC article.
References
-
- World Health Organization Antibiotic Resistance. [(accessed on 24 October 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
-
- Cassini A., Högberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S., Colomb-Cotinat M., Kretzschmar M.E., Devleesschauwer B., Cecchini M., et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. - DOI - PMC - PubMed
-
- Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tu-berculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
-
- Trebosc V., Gartenmann S., Tötzl M., Lucchini V., Schellhorn B., Pieren M., Lociuro S., Gitzinger M., Tigges M., Bumann D., et al. Dissecting Colistin Resistance Mechanisms in Extensively Drug-Resistant Acinetobacter baumannii Clinical Isolates. mBio. 2019;10:e01083-19. doi: 10.1128/mBio.01083-19. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

