The Anti-Virulence Effect of Vismia guianensis against Candida albicans and Candida glabrata
- PMID: 36551490
- PMCID: PMC9774440
- DOI: 10.3390/antibiotics11121834
The Anti-Virulence Effect of Vismia guianensis against Candida albicans and Candida glabrata
Abstract
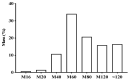
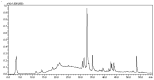
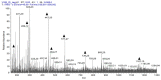
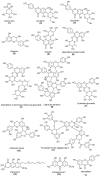
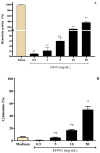
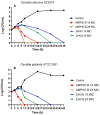
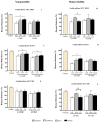
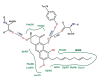
In folk medicine, Vismia guianensis is used to treat skin diseases and mycoses in the Amazon region. We evaluated the anti-Candida activity of the hydroalcoholic extract from the leaves of Vismia guianensis (EHVG). HPLC-PDA and FIA-ESI-IT-MSn were used to chemically characterize EHVG. The anti-Candida activity was determined in vitro by the minimum inhibitory concentrations (MIC) against Candida glabrata (ATCC-2001); Candida albicans (ATCC-90028, ATCC-14053, and ATCC-SC5314), and C. albicans clinical isolates. EHVG effects on adhesion, growth, and biofilm formation were also determined. Molecular docking was used to predict targets for EHVG compounds. The main compounds identified included anthraquinone, vismione D, kaempferol, quercetin, and vitexin. EHVG was fungicidal against all tested strains. C. albicans ATCC 14053 and C. glabrata ATCC 2001 were the most sensitive strains, as the extract inhibited their virulence factors. In silico analysis indicated that vismione D presented the best antifungal activity, since it was the most effective in inhibiting CaCYP51, and may act as anti-inflammatory and antioxidant agent, according to the online PASS prediction. Overall, the data demonstrate that EHVG has an anti-Candida effect by inhibiting virulence factors of the fungi. This activity may be related to its vismione D content, indicating this compound may represent a new perspective for treating diseases caused by Candida sp.
Keywords: CaCYP51; Candida albicans; Candida glabrata; Vismia guianensis; antifungals; lacre; vismione D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Vismia guianensis Improves Survival of Tenebrio molitor and Mice During Lethal Infection with Candida albicans.Antibiotics (Basel). 2025 Jan 11;14(1):72. doi: 10.3390/antibiotics14010072. Antibiotics (Basel). 2025. PMID: 39858358 Free PMC article.
-
Antifungal Activity of Hydroalcoholic Extract of Chrysobalanus icaco Against Oral Clinical Isolates of Candida Species.Pharmacognosy Res. 2017 Jan-Mar;9(1):96-100. doi: 10.4103/0974-8490.199772. Pharmacognosy Res. 2017. PMID: 28250661 Free PMC article.
-
[Investigation of antifungal activity of Ononis spinosa L. ash used for the therapy of skin infections as folk remedies].Mikrobiyol Bul. 2010 Oct;44(4):633-9. Mikrobiyol Bul. 2010. PMID: 21063975 Turkish.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Sertaconazole: a review of its use in the management of superficial mycoses in dermatology and gynaecology.Drugs. 2009;69(3):339-59. doi: 10.2165/00003495-200969030-00009. Drugs. 2009. PMID: 19275277 Review.
Cited by
-
Assessment of Metabolic Alterations Induced by Halogenated Additives and Antifungal Activity of Extracts from the Endophytic Fungus Fusarium sp. Associated with Dizygostemon riparius (Plantaginaceae).Metabolites. 2025 Jul 4;15(7):451. doi: 10.3390/metabo15070451. Metabolites. 2025. PMID: 40710551 Free PMC article.
-
Enhancing Cassava Starch Bioplastics with Vismia guianensis Alcoholic Extract: Characterization with Potential Applications.Polymers (Basel). 2025 Feb 5;17(3):419. doi: 10.3390/polym17030419. Polymers (Basel). 2025. PMID: 39940621 Free PMC article.
-
Antimicrobial and Anti-Infective Activity of Natural Products-Gaining Knowledge from Novel Studies.Antibiotics (Basel). 2023 Jun 15;12(6):1051. doi: 10.3390/antibiotics12061051. Antibiotics (Basel). 2023. PMID: 37370369 Free PMC article.
-
Synthesis of Antimicrobial Chitosan-Silver Nanoparticles Mediated by Reusable Chitosan Fungal Beads.Int J Mol Sci. 2023 Jan 24;24(3):2318. doi: 10.3390/ijms24032318. Int J Mol Sci. 2023. PMID: 36768640 Free PMC article.
-
Effect of Mn(II) and Co(II) on Anti-Candida Metabolite Production by Aspergillus sp. an Endophyte Isolated from Dizygostemon riparius (Plantaginaceae).Pharmaceuticals (Basel). 2024 Dec 12;17(12):1678. doi: 10.3390/ph17121678. Pharmaceuticals (Basel). 2024. PMID: 39770520 Free PMC article.
References
-
- Santos G.C.O., Vasconcelos C.C., Lopes A.J.O., Cartagenes M.S.S., Dualibe Filho A.K., Nascimento F.R.F., Ramos R.M., Pires E.R.R.B., Andrade M.S., Rocha F.M.G., et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front Microbiol. 2018;9:1351. doi: 10.3389/fmicb.2018.01351. - DOI - PMC - PubMed
-
- Santana D.P., Ribeiro E.L., Menezes A.C.S., Naves P.L.F. Novas abordagens sobre os fatores de virulência de Candida albicans. J. Medl. Biol. Sci. 2013;12:229–233.
-
- Lockhart S. Current epidemiology of Candida infection. Clin. Microbiol. News. 2014;36:131–136. doi: 10.1016/j.clinmicnews.2014.08.001. - DOI
LinkOut - more resources
Full Text Sources

