Evolution of RAS Mutations in Cell-Free DNA of Patients with Tissue RAS Wild-Type Metastatic Colorectal Cancer Receiving First-Line Treatment: The PERSEIDA Study
- PMID: 36551560
- PMCID: PMC9776941
- DOI: 10.3390/cancers14246075
Evolution of RAS Mutations in Cell-Free DNA of Patients with Tissue RAS Wild-Type Metastatic Colorectal Cancer Receiving First-Line Treatment: The PERSEIDA Study
Abstract
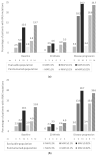
The serial analysis of cell-free DNA (cfDNA) enables minimally invasive monitoring of tumor evolution, providing continuous genetic information. PERSEIDA was an observational, prospective study assessing the cfDNA RAS (KRAS/NRAS) mutational status evolution in first-line, metastatic CRC, RAS wild-type (according to baseline tumor tissue biopsy) patients. Plasma samples were collected before first-line treatment, after 20 ± 2 weeks, and at disease progression. One hundred and nineteen patients were included (102 received panitumumab and chemotherapy as first-line treatment-panitumumab subpopulation). Fifteen (12.6%) patients presented baseline cfDNA RAS mutations (n = 14 [13.7%], panitumumab subpopulation) (mutant allele fraction ≥0.02 for all results). No patients presented emergent mutations (cfDNA RAS mutations not present at baseline) at 20 weeks. At disease progression, 11 patients (n = 9; panitumumab subpopulation) presented emergent mutations (RAS conversion rate: 19.0% [11/58]; 17.7% [9/51], panitumumab subpopulation). In contrast, three (5.2%) patients presenting baseline cfDNA RAS mutations were RAS wild-type at disease progression. No significant associations were observed between overall response rate or progression-free survival and cfDNA RAS mutational status in the total panitumumab subpopulation. Although, in patients with left-sided tumors, a significantly longer progression-free survival was observed in cfDNA RAS wild-type patients compared to those presenting cfDNA RAS mutations at any time. Continuous evaluation of RAS mutations may provide valuable insights on tumor molecular dynamics that can help clinical practice.
Keywords: RAS mutations; cell-free DNA; colorectal cancer; solid biopsy.
Conflict of interest statement
M.V-A. has received grants and personal fees from Roche and personal fees from Merck, Amgen, Sanofi, Servier, Celgene, and Bayer. P.G-A. has received honoraria or consultation fees for speaker, consultancy, or advisory roles from Amgen, Bayer, Bristol, Merck Seorono, MSD, Lilly, Roche, Sanofi, Servier, and Pierre Fabre. R.V.-T. has received speaker fees from Amgen, Merck, Sanofi, Servier, Bristol-MS, Bayer, and Roche and educational and scientific activities and travel support from Amgen, Roche, Lilly, Sanofi, Bristol-MS, Pierre-Fabre, and Servier. R.G-C. has received honoraria for speaker/consulting roles from AAA, Advanz Pharma, Bayer, BMS, HMP, Ipsen, Merck, Midatech Pharma, MSD, Novartis, PharmaMar, Pfizer, Pierre Fabre, Roche, Sanofi, and Servier and research support from ARMO Biosciences, Astrazeneca, Pfizer, Novartis, Ipsen, Roche, Pharmacyclics, Boston Biomedicals, Merck, MSD, Amgen, Sanofi, Bayer, Bristol-Myers-Squibb, Boerhringer, Sysmex, Gilead Sciences, Servier, Adacap, VCN, Lilly, Pharmamar, BMS, and MSD. M-J.S. has received personal fees from Roche, Amgen, Merck, and Sanofi and honoraria for speaker/consulting roles from Amgen, Bayer, BMS, Merck, MSD, Pierre Fabre, Roche, Sanofi, and Servier. JA. has received honoraria for consultancy or advisory roles from Amgen, Merck, Sanofi, Servier, Bayer, and Pierre Fabre. M.S. has received honoraria for speaker and advisory roles for Amgen. A.L.V. is an employee and stakeholder of Amgen S.A. The other authors declare no potential conflicts of interest.
Figures



References
-
- European Commission Colorectal Cancer Burden in EU-27. 2021. [(accessed on 19 May 2022)]. Available online: https://ecis.jrc.ec.europa.eu/pdf/Colorectal_cancer_factsheet-Mar_2021.pdf.
-
- Sociedad Española de Oncología Médica (SEOM) Las Cifras del Cáncer en España 2022. [(accessed on 19 May 2022)]. Available online: https://seom.org/images/LAS_CIFRAS_DEL_CANCER_EN_ESPANA_2022.pdf.
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., van Krieken J.H., Aderka D., Aguilar E.A., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

