Clinical Effect of Lenvatinib Re-Administration after Transcatheter Arterial Chemoembolization in Patients with Intermediate Stage Hepatocellular Carcinoma
- PMID: 36551623
- PMCID: PMC9776720
- DOI: 10.3390/cancers14246139
Clinical Effect of Lenvatinib Re-Administration after Transcatheter Arterial Chemoembolization in Patients with Intermediate Stage Hepatocellular Carcinoma
Abstract
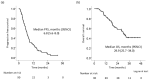
The present study clarified the prognosis of intermediate-stage hepatocellular carcinoma (HCC) patients who received lenvatinib (LEN) followed by transcatheter arterial chemoembolization (TACE) on demand. We retrospectively evaluated 88 intermediate-stage HCC patients who received LEN. The median age was 74 (range: 47-92) years old, 67 patients were male, and 82 were classified as Child-Pugh A. LEN was administered until disease progression or discontinuation due to adverse events (AEs). The mean duration of LEN treatment was 7.0 months. The response and disease control rates were 51.1% and 89.8%, respectively. The median progression-free survival and overall survival (OS) after the initiation of LEN were 6.8 months and 29.9 months, respectively. The OS in patients for whom LEN was re-administered after TACE (TACE-LEN) was better than that in patients who received other therapies (e.g., only TACE, TACE-other therapy, or only other therapy) even with propensity score matching (p = 0.008). A Cox proportional hazard analysis showed that TACE-LEN was most strongly associated with the OS (hazard ratio: 0.083, 95% confidence interval: 0.019-0.362, p = 0.001). LEN was administered for approximately 11.1 months after TACE. In intermediate-stage HCC patients who can tolerate LEN without discontinuation due to AEs, TACE-LEN may prolong the prognosis.
Keywords: TACE; hepatocellular carcinoma (HCC); intermediate stage; lenvatinib; on demand.
Conflict of interest statement
A.I. received the scholarship grants from EISAI Co., Ltd. The company had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The other authors declare no conflict of interest.
Figures



References
-
- National Cancer Center . Center for Cancer Control and Information Services: Cancer Deaths by Demographics. National Cancer Center; Tokyo, Japan: [(accessed on 9 November 2022)]. (In Japanese) Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html.
-
- Bolondi L., Burroughs A., Dufour J.F., Galle P.R., Mazzaferro V., Piscaglia F., Raoul J.L., Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

