Ouabain Effects on Human Anaplastic Thyroid Carcinoma 8505C Cells
- PMID: 36551653
- PMCID: PMC9777381
- DOI: 10.3390/cancers14246168
Ouabain Effects on Human Anaplastic Thyroid Carcinoma 8505C Cells
Abstract
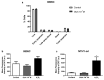
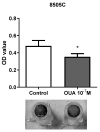
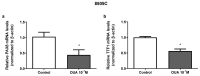
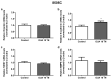
Anaplastic thyroid carcinoma (ATC) is a rare, but aggressive, carcinoma derived from follicular cells. While conventional treatments may improve patients' survival, the lethality remains high. Therefore, there is an urgent need for more effective ATC treatments. Cardiotonic steroids, such as ouabain, have been shown to have therapeutic potential in cancer treatment. Thus, we aimed to evaluate ouabain's effects in human anaplastic thyroid cells. For this, 8505C cells were cultured in the presence or absence of ouabain. Viability, cell death, cell cycle, colony formation and migratory ability were evaluated in ouabain-treated and control 8505C cells. The expression of differentiation and epithelial-to-mesenchymal transition (EMT) markers, as well as IL-6, TGFb1 and their respective receptors were also quantified in these same cells. Our results showed that ouabain in vitro decreased the number of viable 8505C cells, possibly due to an inhibition of proliferation. A reduction in migration was also observed in ouabain-treated 8505C cells. In contrast, decreased mRNA levels of PAX8 and TTF1 differentiation markers and increased levels of the N-cadherin EMT marker, as well as IL-6 and TGFb1, were found in ouabain-treated 8505C cells. In short, ouabain may have anti-proliferative and anti-migratory effect on 8505C cells, but maintains an aggressive and undifferentiated profile.
Keywords: anaplastic thyroid cancer; cardiac glycosides; cardiotonic steroids; cytokines; ouabain.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Molinaro E., Romei C., Biagini A., Sabini E., Agate L., Mazzeo S., Materazzi G., Sellari-Franceschini S., Ribechini A., Torregrossa L., et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017;13:644–660. doi: 10.1038/nrendo.2017.76. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

