Characterization of Glioblastoma Cells Response to Regorafenib
- PMID: 36551679
- PMCID: PMC9777191
- DOI: 10.3390/cancers14246193
Characterization of Glioblastoma Cells Response to Regorafenib
Abstract
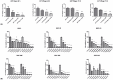
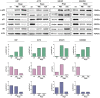
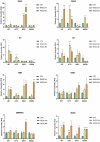
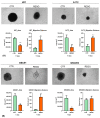
Glioblastoma (GBM), the most malignant primary brain tumor in adults. Although not frequent, it has a relevant social impact because the peak incidence coincides with the age of professional maturity. A number of novel treatments have been proposed, yet clinical trials have been disappointing. Recently, a phase II clinical trial (REGOMA) demonstrated that the multikinase inhibitor regorafenib significantly increased the median overall survival (OS) of GBM patients when compared to lomustine-treated patients. On this basis, the National Comprehensive Cancer Network (NCCN) 2020 Guidelines included regorafenib as a preferred regimen in relapsed GBM treatment. Despite the use in GBM patients' therapy, little is known about the molecular mechanisms governing regorafenib effectiveness on the GBM tumor. Here we report an in vitro characterization of GBM tumor cells' response to regorafenib, performed both on cell lines and on patient-derived glioma stem cells (GSCs). Overall, regorafenib significantly reduced cell growth of 2D tumor cell cultures and of 3D tumor spheroids. Strikingly, this effect was accompanied by transcriptional regulation of epithelial to mesenchymal transition (EMT) genes and by an increased ability of surviving tumor cells to invade the surrounding matrix. Taken together, our data suggest that regorafenib limits cell growth, however, it might induce an invasive phenotype.
Keywords: epithelial to mesenchymal transition (EMT); glioblastoma; glioma stem cells (GSCs); regorafenib; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Reardon D.A., Turner S., Peters K.B., Desjardins A., Gururangan S., Sampson J.H., McLendon R.E., Herndon J.E., Jones L.W., Kirkpatrick J.P., et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J. Natl. Compr. Canc Netw. 2011;9:414–427. doi: 10.6004/jnccn.2011.0038. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

