Factors to Consider for the Correct Use of γH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation
- PMID: 36551689
- PMCID: PMC9776434
- DOI: 10.3390/cancers14246204
Factors to Consider for the Correct Use of γH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation
Abstract
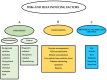
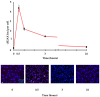
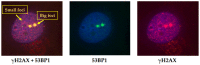
People exposed to ionizing radiation (IR) both for diagnostic and therapeutic purposes is constantly increasing. Since the use of IR involves a risk of harmful effects, such as the DNA DSB induction, an accurate determination of this induced DNA damage and a correct evaluation of the risk-benefit ratio in the clinical field are of key relevance. γH2AX (the phosphorylated form of the histone variant H2AX) is a very early marker of DSBs that can be induced both in physiological conditions, such as in the absence of specific external agents, and by external factors such as smoking, heat, background environmental radiation, and drugs. All these internal and external conditions result in a basal level of γH2AX which must be considered for the correct assessment of the DSBs after IR exposure. In this review we analyze the most common conditions that induce H2AX phosphorylation, including specific exogenous stimuli, cellular states, basic environmental factors, and lifestyles. Moreover, we discuss the most widely used methods for γH2AX determination and describe the principal applications of γH2AX scoring, paying particular attention to clinical studies. This knowledge will help us optimize the use of available methods in order to discern the specific γH2AX following IR-induced DSBs from the basal level of γH2AX in the cells.
Keywords: DNA damage; DNA repair; basal level; double-strand breaks (DSBs); ionizing radiation; γH2AX scoring.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
The loss of gammaH2AX signal is a marker of DNA double strand breaks repair only at low levels of DNA damage.Cell Cycle. 2006 May;5(10):1116-22. doi: 10.4161/cc.5.10.2799. Epub 2006 May 15. Cell Cycle. 2006. PMID: 16721046
-
Most hydrogen peroxide-induced histone H2AX phosphorylation is mediated by ATR and is not dependent on DNA double-strand breaks.J Biochem. 2014 Aug;156(2):85-95. doi: 10.1093/jb/mvu021. Epub 2014 Mar 28. J Biochem. 2014. PMID: 24682951
-
Evaluation of the spatial distribution of gammaH2AX following ionizing radiation.J Vis Exp. 2010 Aug 7;(42):2203. doi: 10.3791/2203. J Vis Exp. 2010. PMID: 20736911 Free PMC article.
-
Persistent γH2AX: A promising molecular marker of DNA damage and aging.Mutat Res Rev Mutat Res. 2015 Oct-Dec;766:1-19. doi: 10.1016/j.mrrev.2015.07.001. Epub 2015 Jul 21. Mutat Res Rev Mutat Res. 2015. PMID: 26596544 Review.
-
Does gammaH2AX foci formation depend on the presence of DNA double strand breaks?Cancer Lett. 2005 Nov 18;229(2):171-9. doi: 10.1016/j.canlet.2005.07.016. Epub 2005 Aug 29. Cancer Lett. 2005. PMID: 16129552 Review.
Cited by
-
The Designer Drug αPHP Affected Cell Proliferation and Triggered Deathly Mechanisms in Murine Neural Stem/Progenitor Cells.Biology (Basel). 2023 Sep 11;12(9):1225. doi: 10.3390/biology12091225. Biology (Basel). 2023. PMID: 37759624 Free PMC article.
-
Robotic radiation shielding system reduces radiation-induced DNA damage in operators performing electrophysiological procedures.Sci Rep. 2025 May 28;15(1):18603. doi: 10.1038/s41598-025-03686-1. Sci Rep. 2025. PMID: 40437055 Free PMC article.
-
Overcoming Multidrug Resistance Using DNA-Localized Auger Emitters: A Comparative Analysis of Radiotoxicity in Breast Cancer Cells.Int J Mol Sci. 2025 Jun 20;26(13):5958. doi: 10.3390/ijms26135958. Int J Mol Sci. 2025. PMID: 40649736 Free PMC article.
-
Cosmic Whirl: Navigating the Comet Trail in DNA: H2AX Phosphorylation and the Enigma of Uncertain Significance Variants.Genes (Basel). 2024 Jun 1;15(6):724. doi: 10.3390/genes15060724. Genes (Basel). 2024. PMID: 38927659 Free PMC article.
-
Hericium erinaceus Extract Exerts Beneficial Effects on Gut-Neuroinflammaging-Cognitive Axis in Elderly Mice.Biology (Basel). 2023 Dec 28;13(1):18. doi: 10.3390/biology13010018. Biology (Basel). 2023. PMID: 38248449 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

