Implementation of Non-Invasive Quantitative Ultrasound in Clinical Cancer Imaging
- PMID: 36551702
- PMCID: PMC9776858
- DOI: 10.3390/cancers14246217
Implementation of Non-Invasive Quantitative Ultrasound in Clinical Cancer Imaging
Abstract
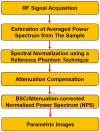
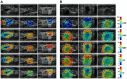
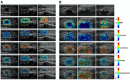
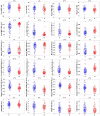
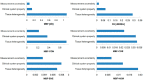
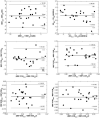
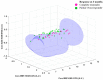
Quantitative ultrasound (QUS) is a non-invasive novel technique that allows treatment response monitoring. Studies have shown that QUS backscatter variables strongly correlate with changes observed microscopically. Increases in cell death result in significant alterations in ultrasound backscatter parameters. In particular, the parameters related to scatterer size and scatterer concentration tend to increase in relation to cell death. The use of QUS in monitoring tumor response has been discussed in several preclinical and clinical studies. Most of the preclinical studies have utilized QUS for evaluating cell death response by differentiating between viable cells and dead cells. In addition, clinical studies have incorporated QUS mostly for tissue characterization, including classifying benign versus malignant breast lesions, as well as responder versus non-responder patients. In this review, we highlight some of the important findings of previous preclinical and clinical studies and expand the applicability and therapeutic benefits of QUS in clinical settings. We summarized some recent clinical research advances in ultrasound-based radiomics analysis for monitoring and predicting treatment response and characterizing benign and malignant breast lesions. We also discuss current challenges, limitations, and future prospects of QUS-radiomics.
Keywords: cell death; chemotherapy; locally advanced breast cancer (LABC); quantitative ultrasound (QUS); radiotherapy; treatment response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sadeghi-Naini A., Papanicolau N., Falou O., Zubovits J., Dent R., Verma S., Trudeau M., Boileau J.F., Spayne J., Iradji S., et al. Quantitative Ultrasound Evaluation of Tumor Cell Death Response in Locally Advanced Breast Cancer Patients Receiving Chemotherapy. Clin. Cancer Res. 2013;19:2163–2174. doi: 10.1158/1078-0432.CCR-12-2965. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

