Natural Compounds in Liposomal Nanoformulations of Potential Clinical Application in Glioblastoma
- PMID: 36551708
- PMCID: PMC9776450
- DOI: 10.3390/cancers14246222
Natural Compounds in Liposomal Nanoformulations of Potential Clinical Application in Glioblastoma
Abstract
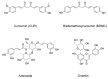
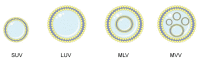
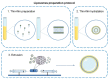
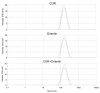
Glioblastoma (GBM) is the most common malignant neoplasm in adults among all CNS gliomas, with the 5-year survival rate being as low as 5%. Among nanocarriers, liposomal nanoformulations are considered as a promising tool for precise drug delivery. The herein presented study demonstrates the possibility of encapsulating four selected natural compounds (curcumin, bisdemethoxycurcumin, acteoside, and orientin) and their mixtures in cationic liposomal nanoformulation composed of two lipid types (DOTAP:POPC). In order to determine the physicochemical properties of the new drug carriers, specific measurements, including particle size, Zeta Potential, and PDI index, were applied. In addition, NMR and EPR studies were carried out for a more in-depth characterization of nanoparticles. Within biological research, the prepared formulations were evaluated on T98G and U-138 MG glioblastoma cell lines in vitro, as well as on a non-cancerous human lung fibroblast cell line (MRC-5) using the MTT test to determine their potential as anticancer agents. The highest activity was exhibited by liposome-entrapped acteoside towards the T98G cell line with IC50 equal 2.9 ± 0.9 µM after 24 hours of incubation. Noteworthy, curcumin and orientin mixture in liposomal formulation exhibited a synergistic effect against GBM. Moreover, the impact on the expression of apoptosis-associated proteins (p53 and Caspase-3) of acteoside as well as curcumin and orientin mixture, as the most potent agents, was assessed, showing nearly 40% increase as compared to control U-138 MG and T98G cells. It should be emphasized that a new and alternative method of extrusion of the studied liposomes was developed.
Keywords: cancer; drug delivery system; glioblastoma; liposomes; natural compounds.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Liposomal Nanoformulation as a Carrier for Curcumin and pEGCG-Study on Stability and Anticancer Potential.Nanomaterials (Basel). 2022 Apr 8;12(8):1274. doi: 10.3390/nano12081274. Nanomaterials (Basel). 2022. PMID: 35457986 Free PMC article.
-
Formulation, development and evaluation of hyaluronic acid-conjugated liposomal nanoparticles loaded with regorafenib and curcumin and their in vitro evaluation on colorectal cancer cell lines.Saudi Pharm J. 2024 Jul;32(7):102099. doi: 10.1016/j.jsps.2024.102099. Epub 2024 May 18. Saudi Pharm J. 2024. PMID: 38817822 Free PMC article.
-
Magnetic cationic liposomal nanocarriers for the efficient drug delivery of a curcumin-based vanadium complex with anticancer potential.J Inorg Biochem. 2019 Oct;199:110778. doi: 10.1016/j.jinorgbio.2019.110778. Epub 2019 Jul 15. J Inorg Biochem. 2019. PMID: 31442839
-
Therapeutic Potential of Curcumin in the Treatment of Glioblastoma Multiforme.Curr Pharm Des. 2019;25(3):333-342. doi: 10.2174/1381612825666190313123704. Curr Pharm Des. 2019. PMID: 30864499 Review.
-
Liposomal-Based Formulations: A Path from Basic Research to Temozolomide Delivery Inside Glioblastoma Tissue.Pharmaceutics. 2022 Jan 27;14(2):308. doi: 10.3390/pharmaceutics14020308. Pharmaceutics. 2022. PMID: 35214041 Free PMC article. Review.
Cited by
-
Potential of Curcumin and Its Analogs in Glioblastoma Therapy.Antioxidants (Basel). 2025 Mar 18;14(3):351. doi: 10.3390/antiox14030351. Antioxidants (Basel). 2025. PMID: 40227413 Free PMC article. Review.
-
Antioxidants Acteoside and Orientin as Emerging Agents in Synergistic Cancer Therapy: A Focus on Innovative Applications.Antioxidants (Basel). 2025 Jul 12;14(7):855. doi: 10.3390/antiox14070855. Antioxidants (Basel). 2025. PMID: 40722959 Free PMC article. Review.
-
Potential Therapeutic Appliances of Dietary Polyphenols: Resveratrol and Curcumin in Treatment of Gliomas.Int J Mol Sci. 2025 Jun 26;26(13):6154. doi: 10.3390/ijms26136154. Int J Mol Sci. 2025. PMID: 40649929 Free PMC article. Review.
-
Glioblastoma Therapy: Past, Present and Future.Int J Mol Sci. 2024 Feb 21;25(5):2529. doi: 10.3390/ijms25052529. Int J Mol Sci. 2024. PMID: 38473776 Free PMC article. Review.
-
CRISPR-Cas9 screening identifies INTS3 as an anti-apoptotic RNA-binding protein and therapeutic target for colorectal cancer.iScience. 2024 Apr 6;27(5):109676. doi: 10.1016/j.isci.2024.109676. eCollection 2024 May 17. iScience. 2024. PMID: 38665208 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

