Quantitative Diffusion-Weighted Imaging Analyses to Predict Response to Neoadjuvant Immunotherapy in Patients with Locally Advanced Head and Neck Carcinoma
- PMID: 36551718
- PMCID: PMC9776484
- DOI: 10.3390/cancers14246235
Quantitative Diffusion-Weighted Imaging Analyses to Predict Response to Neoadjuvant Immunotherapy in Patients with Locally Advanced Head and Neck Carcinoma
Abstract
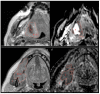
Background: Neoadjuvant immune checkpoint blockade (ICB) prior to surgery may induce early pathological responses in head and neck squamous cell carcinoma (HNSCC) patients. Routine imaging parameters fail to diagnose these responses early on. Magnetic resonance (MR) diffusion-weighted imaging (DWI) has proven to be useful for detecting HNSCC tumor mass after (chemo)radiation therapy.
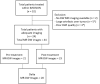
Methods: 32 patients with stage II-IV, resectable HNSCC, treated at a phase Ib/IIa IMCISION trial (NCT03003637), were retrospectively analyzed using MR-imaging before and after two doses of single agent nivolumab (anti-PD-1) (n = 6) or nivolumab with ipilimumab (anti-CTLA-4) ICB (n = 26). The primary tumors were delineated pre- and post-treatment. A total of 32 features were derived from the delineation and correlated with the tumor regression percentage in the surgical specimen.
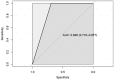
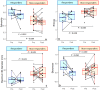
Results: MR-DWI data was available for 24 of 32 patients. Smaller baseline tumor diameter (p = 0.01-0.04) and higher sphericity (p = 0.03) were predictive of having a good pathological response to ICB. Post-treatment skewness and the change in skewness between MRIs were negatively correlated with the tumor's regression (p = 0.04, p = 0.02).
Conclusion: Pre-treatment DWI tumor diameter and sphericity may be quantitative biomarkers for the prediction of an early pathological response to ICB. Furthermore, our data indicate that ADC skewness could be a marker for individual response evaluation.
Keywords: diffusion magnetic resonance imaging; immune checkpoint blockade; immunotherapy; magnetic resonance imaging; radiomics; squamous cell carcinoma of head and neck.
Conflict of interest statement
C.L.Z. is linked to Investigator-initiated clinical trials in collaboration with Bristol Myers Squibb. This funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. All other authors have no conflicts of interest to declare.
Figures

Similar articles
-
Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma.Nat Commun. 2021 Dec 22;12(1):7348. doi: 10.1038/s41467-021-26472-9. Nat Commun. 2021. PMID: 34937871 Free PMC article. Clinical Trial.
-
[18F]FDG-PET accurately identifies pathological response early upon neoadjuvant immune checkpoint blockade in head and neck squamous cell carcinoma.Eur J Nucl Med Mol Imaging. 2022 May;49(6):2010-2022. doi: 10.1007/s00259-021-05610-x. Epub 2021 Dec 27. Eur J Nucl Med Mol Imaging. 2022. PMID: 34957526 Free PMC article. Clinical Trial.
-
Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma.J Immunother Cancer. 2021 May;9(5):e002485. doi: 10.1136/jitc-2021-002485. J Immunother Cancer. 2021. PMID: 33963014 Free PMC article. Clinical Trial.
-
Quantitative diffusion magnetic resonance imaging for prediction of human papillomavirus status in head and neck squamous-cell carcinoma: A systematic review and meta-analysis.Neuroradiol J. 2019 Aug;32(4):232-240. doi: 10.1177/1971400919849808. Epub 2019 May 14. Neuroradiol J. 2019. PMID: 31084347 Free PMC article.
-
Diffusion-weighted Magnetic Resonance Imaging for Predicting Response to Chemoradiation Therapy for Head and Neck Squamous Cell Carcinoma: A Systematic Review.Korean J Radiol. 2019 Apr;20(4):649-661. doi: 10.3348/kjr.2018.0446. Korean J Radiol. 2019. PMID: 30887747 Free PMC article.
Cited by
-
Cancer Immunotherapy and Medical Imaging Research Trends from 2003 to 2023: A Bibliometric Analysis.J Multidiscip Healthc. 2024 May 6;17:2105-2120. doi: 10.2147/JMDH.S457367. eCollection 2024. J Multidiscip Healthc. 2024. PMID: 38736544 Free PMC article. Review.
-
Prediction of pathological complete response in locally advanced head and neck squamous cell carcinoma treated with neoadjuvant chemo-immunotherapy using volumetric multisequence MRI histogram analysis.Neuroradiology. 2024 Jun;66(6):919-929. doi: 10.1007/s00234-024-03339-6. Epub 2024 Mar 20. Neuroradiology. 2024. PMID: 38503986 Clinical Trial.
-
Correction of Gradient Nonlinearity Bias in Apparent Diffusion Coefficient Measurement for Head and Neck Cancers Using Single- and Multi-Shot Echo Planar Diffusion Imaging.Cancers (Basel). 2025 May 28;17(11):1796. doi: 10.3390/cancers17111796. Cancers (Basel). 2025. PMID: 40507277 Free PMC article.
-
Radiomics and PD-L1 expression predict immunotherapy benefits in patients with head and neck squamous cell carcinoma.Future Oncol. 2024;20(36):2869-2878. doi: 10.1080/14796694.2024.2342226. Epub 2024 May 15. Future Oncol. 2024. PMID: 38861311 Free PMC article.
-
A machine learning radiomics based on enhanced computed tomography to predict neoadjuvant immunotherapy for resectable esophageal squamous cell carcinoma.Front Immunol. 2024 Jun 14;15:1405146. doi: 10.3389/fimmu.2024.1405146. eCollection 2024. Front Immunol. 2024. PMID: 38947338 Free PMC article.
References
-
- Rozeman E.A., Hoefsmit E.P., Reijers I.L.M., Saw R.P.M., Versluis J.M., Krijgsman O., Dimitriadis P., Sikorska K., van de Wiel B.A., Eriksson H., et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat. Med. 2021;27:256–263. doi: 10.1038/s41591-020-01211-7. - DOI - PubMed
-
- Du E., Mazul A.L., Farquhar D., Brennan P., Anantharaman D., Abedi-Ardekani B., Weissler M.C., Hayes D.N., Olshan A.F., Zevallos J.P. Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope. 2019;129:2506–2513. doi: 10.1002/lary.27807. - DOI - PMC - PubMed
-
- Bernier J., Domenge C., Ozsahin M., Matuszewska K., Lefèbvre J.-L., Greiner R.H., Giralt J., Maingon P., Rolland F., Bolla M., et al. European Organization for Research and Treatment of Cancer Trial 22931. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. - DOI - PubMed
-
- Seiwert T.Y. ASCO Expanding the Reach of Anti–PD-1 Therapy. Cancer Discov. 2015;5:684–685. doi: 10.1158/2159-8290.cd-nb2015-082. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

