Cutting-Edge CAR Engineering: Beyond T Cells
- PMID: 36551788
- PMCID: PMC9776293
- DOI: 10.3390/biomedicines10123035
Cutting-Edge CAR Engineering: Beyond T Cells
Abstract
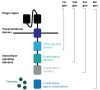
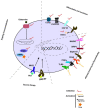
Chimeric antigen receptor (CAR)-T adoptive cell therapy is one of the most promising advanced therapies for the treatment of cancer, with unprecedented outcomes in haematological malignancies. However, it still lacks efficacy in solid tumours, possibly because engineered T cells become inactive within the immunosuppressive tumour microenvironment (TME). In the TME, cells of the myeloid lineage (M) are among the immunosuppressive cell types with the highest tumour infiltration rate. These cells interact with other immune cells, mediating immunosuppression and promoting angiogenesis. Recently, the development of CAR-M cell therapies has been put forward as a new candidate immunotherapy with good efficacy potential. This alternative CAR strategy may increase the efficacy, survival, persistence, and safety of CAR treatments in solid tumours. This remains a critical frontier in cancer research and opens up a new possibility for next-generation personalised medicine to overcome TME resistance. However, the exact mechanisms of action of CAR-M and their effect on the TME remain poorly understood. Here, we summarise the basic, translational, and clinical results of CAR-innate immune cells and CAR-M cell immunotherapies, from their engineering and mechanistic studies to preclinical and clinical development.
Keywords: CAR-NK; CAR-macrophage; CAR-monocyte; CAR-myeloid; immunotherapy; tumour microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- de Erauso L.C., Zuazo M., Arasanz H., Bocanegra A., Hernandez C., Fernandez G., Garcia-Granda M.J., Blanco E., Vera R., Kochan G., et al. Resistance to PD-L1/PD-1 Blockade Immunotherapy. A Tumor-Intrinsic or Tumor-Extrinsic Phenomenon? Front. Pharmacol. 2020;11:441. doi: 10.3389/fphar.2020.00441. - DOI - PMC - PubMed
-
- Hernandez C., Arasanz H., Chocarro L., Bocanegra A., Zuazo M., Fernandez-Hinojal G., Blanco E., Vera R., Escors D., Kochan G. Systemic Blood Immune Cell Populations as Biomarkers for the Outcome of Immune Checkpoint Inhibitor Thera-pies. Int. J. Mol. Sci. 2020;21:2411. doi: 10.3390/ijms21072411. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

