Monoclonal Antibodies and Antivirals against SARS-CoV-2 Reduce the Risk of Long COVID: A Retrospective Propensity Score-Matched Case-Control Study
- PMID: 36551891
- PMCID: PMC9775930
- DOI: 10.3390/biomedicines10123135
Monoclonal Antibodies and Antivirals against SARS-CoV-2 Reduce the Risk of Long COVID: A Retrospective Propensity Score-Matched Case-Control Study
Abstract
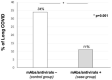
Long COVID is a complex condition affecting quality of life, with limited therapeutic options. We investigated the occurrence of long COVID in subjects receiving early therapy with monoclonal antibodies (mAbs) or antivirals to reduce the risk of COVID-19 progression. In this retrospective study we enrolled 737 adult patients (aged 65.16 ± 13.46; 361F), who experienced COVID-19 between January 2021 and March 2022. Antiviral or mAbs were administered to symptomatic patients who did not require oxygen therapy or hospital admission for SARS-CoV-2 infection, and who were at high risk of progression to severe disease, as identified by age > 65 years or the presence of comorbidities. Long COVID, defined as newly or persistent long-term symptoms 4 weeks after the onset of the acute illness, was reported in 204 cases (28%). Age (OR 1.03; p < 0.001), gender (OR 1.88; p < 0.001) and at least three comorbidities (OR 3.49; p = 0.049) were directly associated with long COVID; conversely, vaccination (OR 0.59; p = 0.005) and mAbs/antivirals (OR 0.44; p = 0.002) were independently associated with a reduced risk of long COVID. At a propensity-score-matched analysis, the mAbs/antivirals group had a significantly lower occurrence of long COVID in comparison with untreated controls (11% vs. 34%; p = 0.001). In conclusion, mAbs and antivirals administered against the progression of COVID-19 were associated with a reduced risk of long COVID.
Keywords: COVID-19; SARS-CoV-2; antivirals; long COVID; monoclonal antibodies; post-COVID syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous