Safety and Tolerability of Oral Cannabinoids in People Living with HIV on Long-Term ART: A Randomized, Open-Label, Interventional Pilot Clinical Trial (CTNPT 028)
- PMID: 36551926
- PMCID: PMC9775551
- DOI: 10.3390/biomedicines10123168
Safety and Tolerability of Oral Cannabinoids in People Living with HIV on Long-Term ART: A Randomized, Open-Label, Interventional Pilot Clinical Trial (CTNPT 028)
Abstract
Background: With anti-inflammatory properties, cannabinoids may be a potential strategy to reduce immune activation in people living with HIV (PLWH) but more information on their safety and tolerability is needed.
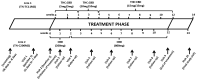
Methods: We conducted an open-label interventional pilot study at the McGill University Health Centre in Montreal, Canada. PLWH were randomized to oral Δ9-tetrahydrocannabinol (THC): cannabidiol (CBD) combination (THC 2.5 mg/CBD 2.5 mg) or CBD-only capsules (CBD 200 mg). Individuals titrated doses as tolerated to a maximum daily dose THC 15 mg/CBD 15 mg or 800 mg CBD, respectively, for 12 weeks. The primary outcome was the percentage of participants without any significant toxicity based on the WHO toxicity scale (Grades 0-2 scores).
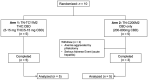
Results: Out of ten individuals, eight completed the study. Two from the CBD-only arm were withdrawn for safety concerns: phlebotomy aggravating pre-existing anemia and severe hepatitis on 800 mg CBD with newly discovered pancreatic adenocarcinoma, respectively. Seven did not have any significant toxicity. Cannabinoids did not alter hematology/biochemistry profiles. CD4 count, CD4/CD8 ratio, and HIV suppression remained stable. Most adverse effects were mild-moderate.
Conclusions: In PLWH, cannabinoids seem generally safe and well-tolerated, though larger studies are needed. Screening for occult liver pathology should be performed and hepatic enzymes monitored, especially with high CBD doses.
Keywords: HIV; cannabidiol (CBD); cannabinoids; chronic liver diseases; pilot clinical trial; quality of life; tetrahydrocannabinol (THC).
Conflict of interest statement
G.S. has acted as speaker for Merck, Gilead, Abbvie, Pfizer, Novonordisk, served as an advisory board member for Merck, Novartis, Pfizer, Gilead and Intercept and has received unrestricted research funding from Theratecnologies. C.T.C. has served on advisory boards for Viiv Healthcare and Gilead, and received grant support from Merck, Gilead, Viiv and Tilray Inc. She has also received travel support to attend conferences from Gilead and Viiv Healthcare.
Figures


References
-
- Cassol E., Malfeld S., Mahasha P., van der Merwe S., Cassol S., Seebregts C., Alfano M., Poli G., Rossouw T. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J. Infect. Dis. 2010;202:723–733. doi: 10.1086/655229. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

