Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets
- PMID: 36551942
- PMCID: PMC9775075
- DOI: 10.3390/biomedicines10123186
Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets
Abstract
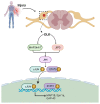
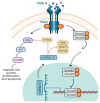
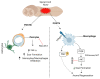
Axons in the peripheral nervous system have the ability to repair themselves after damage, whereas axons in the central nervous system are unable to do so. A common and important characteristic of damage to the spinal cord, brain, and peripheral nerves is the disruption of axonal regrowth. Interestingly, intrinsic growth factors play a significant role in the axonal regeneration of injured nerves. Various factors such as proteomic profile, microtubule stability, ribosomal location, and signalling pathways mark a line between the central and peripheral axons' capacity for self-renewal. Unfortunately, glial scar development, myelin-associated inhibitor molecules, lack of neurotrophic factors, and inflammatory reactions are among the factors that restrict axonal regeneration. Molecular pathways such as cAMP, MAPK, JAK/STAT, ATF3/CREB, BMP/SMAD, AKT/mTORC1/p70S6K, PI3K/AKT, GSK-3β/CLASP, BDNF/Trk, Ras/ERK, integrin/FAK, RhoA/ROCK/LIMK, and POSTN/integrin are activated after nerve injury and are considered significant players in axonal regeneration. In addition to the aforementioned pathways, growth factors, microRNAs, and astrocytes are also commendable participants in regeneration. In this review, we discuss the detailed mechanism of each pathway along with key players that can be potentially valuable targets to help achieve quick axonal healing. We also identify the prospective targets that could help close knowledge gaps in the molecular pathways underlying regeneration and shed light on the creation of more powerful strategies to encourage axonal regeneration after nervous system injury.
Keywords: axonal regeneration; cyclic adenosine monophosphate; microRNAs; nerve injury; neurotrophic factors; regeneration-associated genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Farah M.H., Pan B.H., Hoffman P.N., Ferraris D., Tsukamoto T., Nguyen T., Wong P.C., Price D.L., Slusher B.S., Griffin J.W. Reduced BACE1 Activity Enhances Clearance of Myelin Debris and Regeneration of Axons in the Injured Peripheral Nervous System. J. Neurosci. 2011;31:5744–5754. doi: 10.1523/JNEUROSCI.6810-10.2011. - DOI - PMC - PubMed
-
- Hussain G., Wang J., Rasul A., Anwar H., Qasim M., Zafar S., Aziz N., Razzaq A., Hussain R., de Aguilar J.L.G., et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020;16:116–134. doi: 10.7150/ijbs.35653. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

