Fibrosis-Associated Signaling Molecules Are Differentially Expressed in Palmar Connective Tissues of Patients with Carpal Tunnel Syndrome and Dupuytren's Disease
- PMID: 36551969
- PMCID: PMC9775445
- DOI: 10.3390/biomedicines10123214
Fibrosis-Associated Signaling Molecules Are Differentially Expressed in Palmar Connective Tissues of Patients with Carpal Tunnel Syndrome and Dupuytren's Disease
Abstract
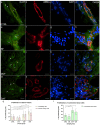
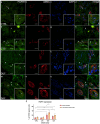
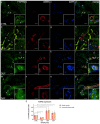
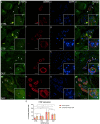
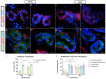
Carpal tunnel syndrome (CTS) and Dupuytren's disease (DD) are fibrotic conditions that affect the connective tissue of the hand and limit its functionality. The exact molecular mechanism underlying the fibrosis is unknown, and only some profibrotic factors have been investigated. In this cross-sectional study, we analyzed the expression of FGF signaling pathway molecules associated with fibrotic changes in the palmar fascia and the flexor retinaculum of 15 CTS patients and both clinically affected and unaffected palmar fascia of 15 DD patients, using immunofluorescence techniques. The expression of FGFR1, FGFR2, and CTGF in the blood vessel walls and surrounding connective tissue cells differed significantly between the analyzed groups, with changes in expression present even in clinically unremarkable tissues from DD patients. We also found altered expression of the analyzed factors, as well as TGF-β1 and syndecan-1 in DD-associated sweat glands, possibly implicating their role in the pathophysiology of the disease. The increased expression of profibrotic factors in the clinically unaffected palmar fascia of DD patients may indicate that more extensive excision is needed during surgical treatment, while the profibrotic factors could be potential targets for developing pharmacological therapeutic strategies against DD-associated fibrosis.
Keywords: CTGF; FGFR1; FGFR2; Syndecan-1; TGF-beta; carpal tunnel syndrome; dupuytren contracture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Investigating the effects of Pirfenidone on TGF-β1 stimulated non-SMAD signaling pathways in Dupuytren's disease -derived fibroblasts.BMC Musculoskelet Disord. 2019 Mar 30;20(1):135. doi: 10.1186/s12891-019-2486-3. BMC Musculoskelet Disord. 2019. PMID: 30927912 Free PMC article.
-
Tamoxifen decreases fibroblast function and downregulates TGF(beta2) in dupuytren's affected palmar fascia.J Surg Res. 2002 Apr;103(2):146-52. doi: 10.1006/jsre.2001.6350. J Surg Res. 2002. PMID: 11922728
-
Chromosomal abnormalities in Dupuytren's contracture and carpal tunnel syndrome.J Hand Surg Br. 1992 Jun;17(3):349-55. doi: 10.1016/0266-7681(92)90128-o. J Hand Surg Br. 1992. PMID: 1624873
-
Concurrent inhibition of TGF-β and mitogen driven signaling cascades in Dupuytren's disease - non-surgical treatment strategies from a signaling point of view.Med Hypotheses. 2012 Mar;78(3):385-8. doi: 10.1016/j.mehy.2011.11.023. Epub 2011 Dec 23. Med Hypotheses. 2012. PMID: 22196988 Review.
-
Blocking fibrotic signaling in fibroblasts from patients with carpal tunnel syndrome.J Cell Physiol. 2018 Mar;233(3):2067-2074. doi: 10.1002/jcp.25901. Epub 2017 May 3. J Cell Physiol. 2018. PMID: 28294324 Free PMC article. Review.
Cited by
-
Musculoskeletal Diseases: From Molecular Basis to Therapy.Biomedicines. 2023 Dec 22;12(1):32. doi: 10.3390/biomedicines12010032. Biomedicines. 2023. PMID: 38255139 Free PMC article.
-
Exploring the potential relationship between frozen shoulder and Dupuytren's disease through bioinformatics analysis and machine learning.Front Immunol. 2023 Aug 31;14:1230027. doi: 10.3389/fimmu.2023.1230027. eCollection 2023. Front Immunol. 2023. PMID: 37720213 Free PMC article.
-
Carpal tunnel syndrome.Nat Rev Dis Primers. 2024 May 23;10(1):37. doi: 10.1038/s41572-024-00521-1. Nat Rev Dis Primers. 2024. PMID: 38782929 Review.
References
-
- Keith M.W., Masear V., Chung K.C., Maupin K., Andary M., Amadio P.C., Watters W.C., 3rd, Goldberg M.J., Haralson R.H., 3rd, Turkelson C.M., et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on diagnosis of carpal tunnel syndrome. J. Bone Jt. Surg. 2009;91:2478–2479. doi: 10.2106/JBJS.I.00643. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

