Local and Systemic Injections of Human Cord Blood Myeloid-Derived Suppressor Cells to Prevent Graft Rejection in Corneal Transplantation
- PMID: 36551981
- PMCID: PMC9776015
- DOI: 10.3390/biomedicines10123223
Local and Systemic Injections of Human Cord Blood Myeloid-Derived Suppressor Cells to Prevent Graft Rejection in Corneal Transplantation
Abstract
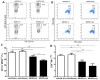
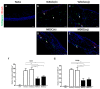
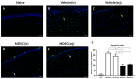
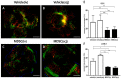
Myeloid-derived suppressor cells (MDSCs) are therapeutic agents to prevent graft rejection in organ transplants by modulating inflammation. Herein, the immunosuppressive effect of human cord blood MDSCs on corneal allograft models was confirmed. CB-MDSCs were locally (subconjuctival, 5 × 105) or systemically (intravenous, 1 × 106) injected twice on days 0 and 7. A corneal transplantation model was established using C57BL/6 and BALB/c mice, and corneal graft opacity was measured to evaluate graft rejection up to 6 weeks. Results showed that graft survival in the MDSCs groups increased compared to vehicle groups after 42 days. Systemic and local MDSC administration inhibited the maturation (MHC-IIhi CD11c+) of dendritic cells (DCs) and the differentiation of interferon γ+ CD4+ Th1 in draining lymph nodes (LNs). However, vehicle groups increased the infiltration of CD3+ T cells and F4/80+ macrophages and produced prominent neovascular and lymphatic vessels into the graft site with increased mRNA expression of VEGF-A/C and VEGFR-1/R-3. Local MDSCs administration showed prominent anti-angiogenic/anti-lymphangiogenic effects even at lower MDSCs doses. Thus, CB-MDSCs could relatively suppress the infiltration of pathological T cells/macrophages into the corneas and the migration of mature DCs into draining LNs Therefore, ocular and systemic MDSCs administration showed therapeutic potential for preventing corneal allograft rejection.
Keywords: Myeloid-derived suppressor cells (MDSCs); T cells; corneal transplantation; graft rejection; macrophages.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Gr-1intCD11b+ myeloid-derived suppressor cells accumulate in corneal allograft and improve corneal allograft survival.J Leukoc Biol. 2016 Dec;100(6):1453-1463. doi: 10.1189/jlb.5A1115-508RR. Epub 2016 Jul 1. J Leukoc Biol. 2016. PMID: 27370015
-
Endogenous Toll-like Receptor 2 Modulates Th1/Treg-Promoting Dendritic Cells in Mice Corneal Transplantation Model.Curr Eye Res. 2020 Jul;45(7):774-781. doi: 10.1080/02713683.2019.1705491. Epub 2019 Dec 26. Curr Eye Res. 2020. PMID: 31842628
-
Blockade of CCR7 leads to decreased dendritic cell migration to draining lymph nodes and promotes graft survival in low-risk corneal transplantation.Exp Eye Res. 2016 May;146:1-6. doi: 10.1016/j.exer.2015.12.004. Epub 2015 Dec 12. Exp Eye Res. 2016. PMID: 26689751
-
Myeloid-derived suppressor cells in transplantation tolerance induction.Int Immunopharmacol. 2020 Jun;83:106421. doi: 10.1016/j.intimp.2020.106421. Epub 2020 Mar 24. Int Immunopharmacol. 2020. PMID: 32217462 Review.
-
Myeloid-Derived Suppressor Cells (MDSC) in the Umbilical Cord Blood: Biological Significance and Possible Therapeutic Applications.J Clin Med. 2022 Jan 29;11(3):727. doi: 10.3390/jcm11030727. J Clin Med. 2022. PMID: 35160177 Free PMC article. Review.
Cited by
-
Local administration of myeloid-derived suppressor cells prevents progression of immune-mediated dry eye disease.Exp Eye Res. 2024 May;242:109871. doi: 10.1016/j.exer.2024.109871. Epub 2024 Mar 26. Exp Eye Res. 2024. PMID: 38527580 Free PMC article.
-
Serum Extracellular Vesicle Protein Profiling for Prediction of Corneal Transplant Rejection.Transplantation. 2024 Jun 1;108(6):1368-1375. doi: 10.1097/TP.0000000000004946. Epub 2024 Feb 27. Transplantation. 2024. PMID: 38409732 Free PMC article.
-
The Role of Protein Kinases in the Suppressive Phenotype of Myeloid-Derived Suppressor Cells.Int J Mol Sci. 2025 Jul 19;26(14):6936. doi: 10.3390/ijms26146936. Int J Mol Sci. 2025. PMID: 40725182 Free PMC article. Review.
-
Local Myeloid-Derived Suppressor Cells Impair Progression of Experimental Autoimmune Uveitis by Alleviating Oxidative Stress and Inflammation.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):39. doi: 10.1167/iovs.64.13.39. Invest Ophthalmol Vis Sci. 2023. PMID: 37878302 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

