Fourth Dose of mRNA COVID-19 Vaccine Transiently Reactivates Spike-Specific Immunological Memory in People Living with HIV (PLWH)
- PMID: 36552017
- PMCID: PMC9775459
- DOI: 10.3390/biomedicines10123261
Fourth Dose of mRNA COVID-19 Vaccine Transiently Reactivates Spike-Specific Immunological Memory in People Living with HIV (PLWH)
Abstract
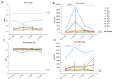
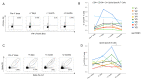
Background: People Living With HIV (PLWH), with advanced disease, lower CD4+ T cell counts or an unsuppressed HIV viral load can have a suboptimal vaccine response. For this reason, in the current COVID-19 pandemic, they represent a prioritized population for the SARS-CoV-2 fourth (or second booster) vaccine dose. This work aims to investigate the effects of a second booster on the reactivation of the spike-specific humoral and cell-mediated immune responses in PLWH. Methods: A total of eight PLWH, who received a fourth dose of the original mRNA vaccines were enrolled. They were evaluated before and then 7 days, 1 month and 2 months after the injection. The humoral response was assessed via a chemiluminescent immunoassay. Immunophenotyping and the functional evaluation of the SARS-CoV-2-specific cellular immune responses were performed via flow cytometry. Results: Anti-spike IgG levels were above the cut-off value for all subjects at all timepoints. The spike-specific CD4+ T cell response was reactivated one week after the fourth vaccine dose, and on average declined at two months post-vaccination. A similar trend was observed for the spike-specific B cells. A low percentage of spike-specific CD4+ T cells was activated by the B.1.1.529 BA.1 Omicron-spike mutated peptides, and the majority of these cells were reactive to the conserved portions of the spike protein. Similarly, the majority of the spike-specific memory B cells were able to bind both Wuhan and Omicron-spike entire protein. Conclusions: Spike-specific adaptive immune responses are transiently reactivated in PLWH following the fourth mRNA vaccine dose. The breadth of the immune responses to the mutated spike protein provides insight on the possible cross-reactivity for the SARS-CoV-2 variants of concern (VOCs).
Keywords: B cell response; HIV; Omicron; SARS-CoV-2; T cell response; fourth dose; humoral response; mRNA vaccine; people living with HIV.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Long-term immune responses to SARS-CoV-2 Omicron BA.4/5 mRNA booster in people living with HIV.Commun Med (Lond). 2025 Mar 27;5(1):92. doi: 10.1038/s43856-025-00799-6. Commun Med (Lond). 2025. PMID: 40148493 Free PMC article.
-
The humoral and cellular immune responses following booster vaccination with SARS-CoV-2 mRNA in people living with human immunodeficiency virus.J Infect Chemother. 2024 May;30(5):417-422. doi: 10.1016/j.jiac.2023.11.014. Epub 2023 Nov 16. J Infect Chemother. 2024. PMID: 37977325
-
Relatively preserved functional immune capacity with standard COVID-19 vaccine regimen in people living with HIV.Front Immunol. 2023 Sep 4;14:1204314. doi: 10.3389/fimmu.2023.1204314. eCollection 2023. Front Immunol. 2023. PMID: 37731482 Free PMC article.
-
Immune responses to mRNA-based vaccines given as a third COVID-19 vaccine dose in people living with HIV-a literature review.APMIS. 2024 Apr;132(4):236-244. doi: 10.1111/apm.13379. Epub 2024 Jan 26. APMIS. 2024. PMID: 38275143 Review.
-
Immunogenicity and effectiveness of COVID-19 booster vaccination among people living with HIV: a systematic review and meta-analysis.Front Med (Lausanne). 2023 Oct 9;10:1275843. doi: 10.3389/fmed.2023.1275843. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37877024 Free PMC article.
Cited by
-
Third COVID-19 vaccine dose boosts antibody function in Rwandans with high HIV viral load.iScience. 2023 Sep 19;26(10):107959. doi: 10.1016/j.isci.2023.107959. eCollection 2023 Oct 20. iScience. 2023. PMID: 37810226 Free PMC article.
-
Immunogenicity and Determinants of Antibody Response to the BNT162b2 mRNA Vaccine: A Longitudinal Study in a Cohort of People Living with HIV.Vaccines (Basel). 2024 Oct 16;12(10):1172. doi: 10.3390/vaccines12101172. Vaccines (Basel). 2024. PMID: 39460338 Free PMC article.
-
Severe acute respiratory syndrome coronavirus 2-specific T-cell responses are induced in people living with human immunodeficiency virus after booster vaccination.Chin Med J (Engl). 2024 Nov 20;137(22):2734-2744. doi: 10.1097/CM9.0000000000003176. Epub 2024 Jul 18. Chin Med J (Engl). 2024. PMID: 39028115 Free PMC article.
-
Mitochondrial Dysfunction in Cardiotoxicity Induced by BCR-ABL1 Tyrosine Kinase Inhibitors -Underlying Mechanisms, Detection, Potential Therapies.Cardiovasc Toxicol. 2023 Aug;23(7-8):233-254. doi: 10.1007/s12012-023-09800-x. Epub 2023 Jul 21. Cardiovasc Toxicol. 2023. PMID: 37479951 Review.
-
Humoral response after a fourth dose of SARS-CoV-2 vaccine in immunocompromised patients. Results of a systematic review.Front Public Health. 2023 Mar 23;11:1108546. doi: 10.3389/fpubh.2023.1108546. eCollection 2023. Front Public Health. 2023. PMID: 37033069 Free PMC article.
References
-
- Nomah D.K., Reyes-Urueña J., Llibre J.M., Ambrosioni J., Ganem F.S., Miró J.M., Casabona J. HIV and SARS-CoV-2 Co-infection: Epidemiological, Clinical Features, and Future Implications for Clinical Care and Public Health for People Living with HIV (PLWH) and HIV Most-at-Risk Groups. Curr. HIV/AIDS Rep. 2021;18:518–526. doi: 10.1007/s11904-021-00579-6. - DOI - PMC - PubMed
-
- Chu C., Pollock L.C., Selwyn P.A. HIV-Associated Complications: A Systems-Based Approach. Am. Fam. Physician. 2017;96:161–169. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

