Fourth Dose of mRNA COVID-19 Vaccine Transiently Reactivates Spike-Specific Immunological Memory in People Living with HIV (PLWH)
- PMID: 36552017
- PMCID: PMC9775459
- DOI: 10.3390/biomedicines10123261
Fourth Dose of mRNA COVID-19 Vaccine Transiently Reactivates Spike-Specific Immunological Memory in People Living with HIV (PLWH)
Abstract
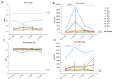
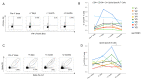
Background: People Living With HIV (PLWH), with advanced disease, lower CD4+ T cell counts or an unsuppressed HIV viral load can have a suboptimal vaccine response. For this reason, in the current COVID-19 pandemic, they represent a prioritized population for the SARS-CoV-2 fourth (or second booster) vaccine dose. This work aims to investigate the effects of a second booster on the reactivation of the spike-specific humoral and cell-mediated immune responses in PLWH. Methods: A total of eight PLWH, who received a fourth dose of the original mRNA vaccines were enrolled. They were evaluated before and then 7 days, 1 month and 2 months after the injection. The humoral response was assessed via a chemiluminescent immunoassay. Immunophenotyping and the functional evaluation of the SARS-CoV-2-specific cellular immune responses were performed via flow cytometry. Results: Anti-spike IgG levels were above the cut-off value for all subjects at all timepoints. The spike-specific CD4+ T cell response was reactivated one week after the fourth vaccine dose, and on average declined at two months post-vaccination. A similar trend was observed for the spike-specific B cells. A low percentage of spike-specific CD4+ T cells was activated by the B.1.1.529 BA.1 Omicron-spike mutated peptides, and the majority of these cells were reactive to the conserved portions of the spike protein. Similarly, the majority of the spike-specific memory B cells were able to bind both Wuhan and Omicron-spike entire protein. Conclusions: Spike-specific adaptive immune responses are transiently reactivated in PLWH following the fourth mRNA vaccine dose. The breadth of the immune responses to the mutated spike protein provides insight on the possible cross-reactivity for the SARS-CoV-2 variants of concern (VOCs).
Keywords: B cell response; HIV; Omicron; SARS-CoV-2; T cell response; fourth dose; humoral response; mRNA vaccine; people living with HIV.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nomah D.K., Reyes-Urueña J., Llibre J.M., Ambrosioni J., Ganem F.S., Miró J.M., Casabona J. HIV and SARS-CoV-2 Co-infection: Epidemiological, Clinical Features, and Future Implications for Clinical Care and Public Health for People Living with HIV (PLWH) and HIV Most-at-Risk Groups. Curr. HIV/AIDS Rep. 2021;18:518–526. doi: 10.1007/s11904-021-00579-6. - DOI - PMC - PubMed
-
- Chu C., Pollock L.C., Selwyn P.A. HIV-Associated Complications: A Systems-Based Approach. Am. Fam. Physician. 2017;96:161–169. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

