Acute Effects of High-Frequency Insular Stimulation on Interictal Epileptiform Discharge Rates in Patients with Refractory Epilepsy
- PMID: 36552076
- PMCID: PMC9775111
- DOI: 10.3390/brainsci12121616
Acute Effects of High-Frequency Insular Stimulation on Interictal Epileptiform Discharge Rates in Patients with Refractory Epilepsy
Abstract
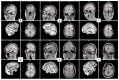
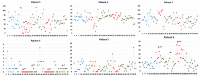
Rationale: Deep brain stimulation (DBS) of several sites, such as the thalamus, has been shown to reduce seizure frequency and interictal epileptiform activity in patients with refractory epilepsy. Recent findings have demonstrated that the insula is part of the ‘rich club’ of highly connected brain regions. This pilot study investigated short-term effects of high-frequency (HF) insular DBS on interictal epileptiform discharge (IED) rate in patients with refractory epilepsy. Methods: Six patients with drug-resistant epilepsy undergoing an intracranial electroencephalographic study received two sets of 10 min continuous 150 Hz HF-DBS of the insula. For each patient, epileptiform activity was analyzed for a total of 80 min, starting 20 min prior to stimulation set 1 (S1), and ending 20 min after stimulation set 2 (S2). All IEDs were identified and classified according to their anatomic localization by a board-certified epileptologist. The IED rate during the 20 min preceding S1 served as a baseline for comparison with IED rate during S1, S2 and post-stimulation periods. Results: HF-DBS of the anterior insula (aINS) was performed in a patient with an aINS epileptic focus (patient 1). HF-DBS of the posterior insula (pINS) was performed in two patients with a pINS epileptic focus (patients 2 and 4), in one patient with an aINS focus (patient 3), and in two non-insular patients (patients 5 and 6). The total IED (irrespective of their location) rate significantly decreased (p < 0.01) in two patients (patients 1 and 2) during the stimulation period, whereas it significantly increased (p < 0.01) in one patient (patient 6); there was no change in the other three patients. Looking at subsets of spike localization, HF-DBS of the aINS significantly reduced aINS and orbitofrontal IEDs in patient 1 (p < 0.01), while HF-DBS of the pINS had an effect on pINS IEDs (p < 0.01) in both patients with a pINS focus; there was no significant effect of HF-DBS of the insula on IEDs in temporal or other frontal regions. Conclusion: Short-term HF-DBS of the insula had heterogeneous effects on the IED rate. Further work is required to examine factors underlying these heterogeneous effects, such as stimulation frequency, location of IEDs and subregions of the insula stimulated.
Keywords: deep brain stimulation; high-frequency stimulation; insular epilepsy; interictal epileptiform discharge frequency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Salanova V., Witt T., Worth R., Henry T.R., Gross R.E., Nazzaro J.M., Labar D., Sperling M.R., Sharan A., Sandok E., et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. doi: 10.1212/WNL.0000000000001334. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

