Common Data Elements Reported in Mechanical Thrombectomy for Acute Ischemic Stroke: A Systematic Review of Active Clinical Trials
- PMID: 36552140
- PMCID: PMC9775042
- DOI: 10.3390/brainsci12121679
Common Data Elements Reported in Mechanical Thrombectomy for Acute Ischemic Stroke: A Systematic Review of Active Clinical Trials
Abstract
Background: New trials are planned regularly to provide the highest quality of evidence and invade new occlusion territories, which requires a pre-defined reporting strategy with consistent, common data elements for more straightforward collective evidence synthesis. We sought to review all active endovascular thrombectomy trials to investigate their patient selection criteria, intervention description, and reported outcomes.
Methods: A literature search was systematically conducted on clinicaltrials.gov for active trials and all intervention, inclusion criteria, and outcomes reported were extracted. A qualitative synthesis of the frequency of study design types and data elements are graphically and narratively presented.
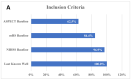
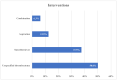
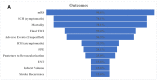
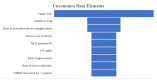
Results: A total of 32 studies were tagged and included in the final qualitative analysis. The inclusion criteria were highly variable, including different cut-offs for the last well-known baseline National Institutes of Health Stroke Scale, Alberta Stroke Program Early CT Score, and modified Rankin scale (mRS). Half of the studies (16/32) mentioned "thrombectomy" without defining which technique or device was used, and the final thrombolysis in cerebral infarction scale was provided in 19 (59.4%) studies. Heterogeneity was also present among the studies reporting a first-pass effect, both in how studies defined the outcome and in used ranges for mRS. Mortality and intracerebral hemorrhage (ICH) were more homogenous in their presentation and follow-up.
Conclusions: There is a great degree of heterogeneity in the active thrombectomy trials concerning inclusion criteria, interventions used, and how outcomes are being reported.
Keywords: clinical Trial; common data elements; stroke; thrombectomy.
Conflict of interest statement
K.M.K. works for and holds equity in Nested Knowledge, Inc., works for Conway Medical LLC, and holds equity in Superior Medical Experts, Inc. D.F.K. has the following conflicts: ownership in Nested Knowledge, Inc., Superior Medical Experts, Inc., Conway Medical LLC; research support from Microvention, Balt USA, Medtronic.
Figures


References
-
- Feigin V.L., Stark B.A., Johnson C.O., Roth G.A., Bisignano C., Abady G.G., Abbasifard M., Abbasi-Kangevari M., Abd-Allah F., Abedi V., et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. - DOI - PMC - PubMed
-
- Albers G.W., Marks M.P., Kemp S., Christensen S., Tsai J.P., Ortega-Gutierrez S., McTaggart R.A., Torbey M.T., Kim-Tenser M., Leslie-Mazwi T., et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. - DOI - PMC - PubMed
-
- Dhillon P.S., Butt W., Podlasek A., McConachie N., Lenthall R., Nair S., Malik L., Bhogal P., Makalanda H.L.D., Spooner O., et al. Association between time to treatment and clinical outcomes in endovascular thrombectomy beyond 6 hours without advanced imaging selection. J. Neurointerv. Surg. 2022 doi: 10.1136/neurintsurg-2021-018564. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

