Depression-Associated Negr1 Gene-Deficiency Induces Alterations in the Monoaminergic Neurotransmission Enhancing Time-Dependent Sensitization to Amphetamine in Male Mice
- PMID: 36552158
- PMCID: PMC9776224
- DOI: 10.3390/brainsci12121696
Depression-Associated Negr1 Gene-Deficiency Induces Alterations in the Monoaminergic Neurotransmission Enhancing Time-Dependent Sensitization to Amphetamine in Male Mice
Abstract
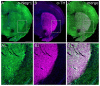
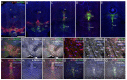
In GWAS studies, the neural adhesion molecule encoding the neuronal growth regulator 1 (NEGR1) gene has been consistently linked with both depression and obesity. Although the linkage between NEGR1 and depression is the strongest, evidence also suggests the involvement of NEGR1 in a wide spectrum of psychiatric conditions. Here we show the expression of NEGR1 both in tyrosine- and tryptophan hydroxylase-positive cells. Negr1-/- mice show a time-dependent increase in behavioral sensitization to amphetamine associated with increased dopamine release in both the dorsal and ventral striatum. Upregulation of transcripts encoding dopamine and serotonin transporters and higher levels of several monoamines and their metabolites was evident in distinct brain areas of Negr1-/- mice. Chronic (23 days) escitalopram-induced reduction of serotonin and dopamine turnover is enhanced in Negr1-/- mice, and escitalopram rescued reduced weight of hippocampi in Negr1-/- mice. The current study is the first to show alterations in the brain monoaminergic systems in Negr1-deficient mice, suggesting that monoaminergic neural circuits contribute to both depressive and obesity-related phenotypes linked to the human NEGR1 gene.
Keywords: Negr1; depression; dopamine; genetic models; serotonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Neural cell adhesion molecule Negr1 deficiency in mouse results in structural brain endophenotypes and behavioral deviations related to psychiatric disorders.Sci Rep. 2019 Apr 1;9(1):5457. doi: 10.1038/s41598-019-41991-8. Sci Rep. 2019. PMID: 30932003 Free PMC article.
-
Neuronal Growth and Behavioral Alterations in Mice Deficient for the Psychiatric Disease-Associated Negr1 Gene.Front Mol Neurosci. 2018 Feb 9;11:30. doi: 10.3389/fnmol.2018.00030. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29479305 Free PMC article.
-
Functional inactivation of the genome-wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype.PLoS One. 2012;7(7):e41537. doi: 10.1371/journal.pone.0041537. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844493 Free PMC article.
-
From Bound Cells Comes a Sound Mind: The Role of Neuronal Growth Regulator 1 in Psychiatric Disorders.Exp Neurobiol. 2020 Feb 29;29(1):1-10. doi: 10.5607/en.2020.29.1.1. Exp Neurobiol. 2020. PMID: 32122104 Free PMC article. Review.
-
[Consequences of the monoaminergic systems cross-talk in the antidepressant activity].Encephale. 2018 Jun;44(3):264-273. doi: 10.1016/j.encep.2018.05.001. Epub 2018 May 22. Encephale. 2018. PMID: 29801770 Review. French.
Cited by
-
Is There a Natural, Non-addictive, and Non-anti-reward, Safe, Gene-based Solution to Treat Reward Deficiency Syndrome? KB220 Variants vs GLP-1 Analogs.J Addict Psychiatry. 2024;8(1):34-49. Epub 2024 May 20. J Addict Psychiatry. 2024. PMID: 39618491 Free PMC article.
-
The Role of IgLON Cell Adhesion Molecules in Neurodegenerative Diseases.Genes (Basel). 2023 Sep 28;14(10):1886. doi: 10.3390/genes14101886. Genes (Basel). 2023. PMID: 37895235 Free PMC article. Review.
-
The IgLON family of cell adhesion molecules expressed in developing neural circuits ensure the proper functioning of the sensory system in mice.Sci Rep. 2024 Sep 30;14(1):22593. doi: 10.1038/s41598-024-73358-z. Sci Rep. 2024. PMID: 39349721 Free PMC article.
-
Dissecting shared genetic architecture between depression and body mass index.BMC Med. 2024 Oct 11;22(1):455. doi: 10.1186/s12916-024-03681-9. BMC Med. 2024. PMID: 39394142 Free PMC article.
References
-
- Hyde C.L., Nagle M.W., Tian C., Chen X., Paciga S.A., Wendland J.R., Tung J.Y., Hinds D.A., Perlis R.H., Winslow A.R. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 2016;48:1031–1036. doi: 10.1038/ng.3623. - DOI - PMC - PubMed
-
- Wray N.R., Ripke S., Mattheisen M., Trzaskowski M., Byrne E.M., Abdel A., Adams M.J., Agerbo E., Air T.M., Andlauer T.M.F., et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. - DOI - PMC - PubMed
-
- Howard D.M., Adams M.J., Shirali M., Clarke T.K., Marioni R.E., Davies G., Coleman J.R.I., Alloza C., Shen X., Barbu M.C., et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018;9:1470. doi: 10.1038/s41467-018-03819-3. - DOI - PMC - PubMed
-
- Howard D.M., Adams M.J., Clarke T.K., Hafferty J.D., Gibson J., Shirali M., Coleman J.R.I., Hagenaars S.P., Ward J., Eigmore E.M., et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019;22:343–352. doi: 10.1038/s41593-018-0326-7. - DOI - PMC - PubMed
-
- Levey D.F., Stein M.B., Wendt F.R., Pathak G.A., Zhou H., Aslan M., Quaden R., Harrington K.M., Nuñez Y.Z., Overstreet C., et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 2021;24:954–963. doi: 10.1038/s41593-021-00860-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

