PIP2 Interacts Electrostatically with MARCKS-like Protein-1 and ENaC in Renal Epithelial Cells
- PMID: 36552204
- PMCID: PMC9774185
- DOI: 10.3390/biology11121694
PIP2 Interacts Electrostatically with MARCKS-like Protein-1 and ENaC in Renal Epithelial Cells
Abstract
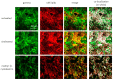
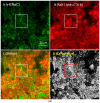
We examined the interaction of a membrane-associated protein, MARCKS-like Protein-1 (MLP-1), and an ion channel, Epithelial Sodium Channel (ENaC), with the anionic lipid, phosphatidylinositol 4, 5-bisphosphate (PIP2). We found that PIP2 strongly activates ENaC in excised, inside-out patches with a half-activating concentration of 21 ± 1.17 µM. We have identified 2 PIP2 binding sites in the N-terminus of ENaC β and γ with a high concentration of basic residues. Normal channel activity requires MLP-1's strongly positively charged effector domain to electrostatically sequester most of the membrane PIP2 and increase the local concentration of PIP2. Our previous data showed that ENaC covalently binds MLP-1 so PIP2 bound to MLP-1 would be near PIP2 binding sites on the cytosolic N terminal regions of ENaC. We have modified the charge structure of the PIP2 -binding domains of MLP-1 and ENaC and showed that the changes affect membrane localization and ENaC activity in a way consistent with electrostatic theory.
Keywords: ENaC; MARCKS-like protein-1; MARCKS-like-1; PIP2; mpkCCD cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Myristoylated alanine-rich C kinase substrate-like protein-1 regulates epithelial sodium channel activity in renal distal convoluted tubule cells.Am J Physiol Cell Physiol. 2020 Sep 1;319(3):C589-C604. doi: 10.1152/ajpcell.00218.2020. Epub 2020 Jul 8. Am J Physiol Cell Physiol. 2020. PMID: 32639874 Free PMC article.
-
Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane.Am J Physiol Renal Physiol. 2015 Sep 1;309(5):F456-63. doi: 10.1152/ajprenal.00631.2014. Epub 2015 Jul 1. Am J Physiol Renal Physiol. 2015. PMID: 26136560 Free PMC article.
-
Phosphatidylinositol 4,5-bisphosphate directly interacts with the β and γ subunits of the sodium channel ENaC.J Biol Chem. 2020 Jun 5;295(23):7958-7969. doi: 10.1074/jbc.RA120.012606. Epub 2020 Apr 27. J Biol Chem. 2020. PMID: 32341072 Free PMC article.
-
Acute regulation of epithelial sodium channel by anionic phospholipids.J Am Soc Nephrol. 2005 Nov;16(11):3182-7. doi: 10.1681/ASN.2005040434. Epub 2005 Sep 28. J Am Soc Nephrol. 2005. PMID: 16192420 Review.
-
Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides.Am J Physiol Renal Physiol. 2006 May;290(5):F949-57. doi: 10.1152/ajprenal.00386.2005. Am J Physiol Renal Physiol. 2006. PMID: 16601296 Review.
Cited by
-
The Epithelial Sodium Channel-An Underestimated Drug Target.Int J Mol Sci. 2023 Apr 24;24(9):7775. doi: 10.3390/ijms24097775. Int J Mol Sci. 2023. PMID: 37175488 Free PMC article. Review.
-
Membrane Surface Charge, Phospholipids, and Protein Localization.Rev Physiol Biochem Pharmacol. 2025;187:89-101. doi: 10.1007/978-3-031-68827-0_9. Rev Physiol Biochem Pharmacol. 2025. PMID: 39838010 Review.
-
SECS, drugs, and Rac1&Rho: regulation of EnNaC in vascular endothelial cells.Pflugers Arch. 2025 Jul;477(7):977-992. doi: 10.1007/s00424-025-03093-5. Epub 2025 May 22. Pflugers Arch. 2025. PMID: 40402207 Free PMC article.
-
Augmentation of Cathepsin Isoforms in Diabetic db/db Mouse Kidneys Is Associated with an Increase in Renal MARCKS Expression and Proteolysis.Int J Mol Sci. 2023 Aug 5;24(15):12484. doi: 10.3390/ijms241512484. Int J Mol Sci. 2023. PMID: 37569859 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

