A New Cell Line Derived from the Spleen of the Japanese Flounder (Paralichthys olivaceus) and Its Application in Viral Study
- PMID: 36552207
- PMCID: PMC9774307
- DOI: 10.3390/biology11121697
A New Cell Line Derived from the Spleen of the Japanese Flounder (Paralichthys olivaceus) and Its Application in Viral Study
Abstract
A new cell line Japanese flounder spleen (JFSP) derived from the spleen of Japanese flounder (Paralichthys olivaceus) was established and characterized in this study. The JFSP cells grew rapidly at 29 °C, and the optimum fetal bovine serum concentration in the L-15 medium was 15%. Cells were subcultured for more than 80 passages. The JFSP cells have a diploid chromosome number of 2n = 68, which differs from the chromosome number of normal diploid Japanese flounder. The established cells were susceptible to Bohle virus (BIV), Viral hemorrhagic septicemia virus (VHSV), Hirame rhabdovirus (HIRRV), Infectious hematopoietic necrosis virus (IHNV), and Lymphocystis disease virus (LCDV), as evidenced by varying degrees of cytopathic effects (CPE). Replication of the virus in JFSP cells was confirmed by qRT-PCR and transmission electron microscopy. In addition, the expression of four immune-related genes, TRAF3, IL-1β, TNF-α, and TLR2, was differentially altered following viral infection. The results indicated that the cells underwent an antiviral immune response. JFSP cell line is an ideal tool in vitro for virology. The use of fish cell lines to study the immune genes and immune mechanism of fish and to clarify the immune mechanism of fish has important theoretical significance and practical application value for the fundamental prevention and treatment of fish diseases.
Keywords: Bohle virus; Lymphocystis disease virus; Paralichthys olivaceus; cell line; cell transfection; virus susceptibility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

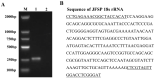




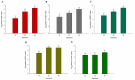


References
-
- Guo Y.N., Nan X.Y., Zhang X.Y., Wang G.X., Ren Y.Q., Wang Y.F., Fu Y.S., Hou J.L. Molecular characterization and functional analysis of Japanese flounder (Paralichthys olivaceus) thbs2 in response to lymphocystis disease virus. Fish Shellfish Immunol. 2019;93:183–190. doi: 10.1016/j.fsi.2019.07.055. - DOI - PubMed
-
- Kim W.S., Kim S.R., Kim D., Kim J.O., Park M.A., Kitamura S.I., Kim H.Y., Kim D.H., Han H.J., Jung S.J., et al. An outbreak of VHSV (viral hemorrhagic septicemia virus) infection in farmed olive flounder Paralichthys olivaceus in Korea. Aquaculture. 2009;296:165–168. doi: 10.1016/j.aquaculture.2009.07.019. - DOI
-
- Gong C.G., Zhang Y.T., Wang G.X., Liu Y.F., He Z.W., Ren Y.Q., Cao W., Zhao H.T., Xu Y.H., Wang Y.F., et al. The isolation and full-length transcriptome sequencing of a novel nidovirus and response of its infection in Japanese flounder (Paralichthys olivaceus) Viruses. 2022;14:1216. doi: 10.3390/v14061216. - DOI - PMC - PubMed
Grants and funding
- 2022TD38/Central Public-Interest Scientific Institution Basal Research Fund, CAFS
- C2021107002/Nature Science Foundation of Hebei Province, China
- C2022107003/Nature Science Foundation of Hebei Province, China
- 21326307D/the Key R&D Program of Hebei Province, China
- CARS-47/The National Marine Genetic Resource Center, and China Agriculture Research System
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

