Alleviation of Cognitive and Physical Fatigue with Enzymatic Porcine Placenta Hydrolysate Intake through Reducing Oxidative Stress and Inflammation in Intensely Exercised Rats
- PMID: 36552249
- PMCID: PMC9774658
- DOI: 10.3390/biology11121739
Alleviation of Cognitive and Physical Fatigue with Enzymatic Porcine Placenta Hydrolysate Intake through Reducing Oxidative Stress and Inflammation in Intensely Exercised Rats
Abstract
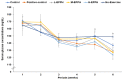
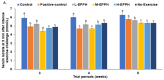
Intense exercise is reported to induce physical and cognitive fatigue, but few studies have focused on treatments to alleviate fatigue. We hypothesized that the oral supplementation of enzymatic porcine placenta hydrolysate (EPPH) prepared using protease enzymes could alleviate exercise-induced fatigue in an animal model. The objectives of the study were to examine the hypothesis and the action mechanism of EPPH in relieving physical and cognitive fatigue. Fifty male Sprague−Dawley rats aged 8 weeks (body weight: 201 g) were classified into five groups, and rats in each group were given oral distilled water, EPPH (5 mg nitrogen/mL) at doses of 0.08, 0.16, or 0.31 mL/kg body weight (BW)/day, or glutathione (100 mg/kg BW/day) by a feeding needle for 5 weeks, which were named as the control, L-EPPH, M-EPPH, H-EPPH, or positive-control groups, respectively. Ten additional rats had no intense exercise with water administration and were designated as the no-exercise group. After 2 weeks, the rats were subjected to intense exercise and forced swimming trial for 30 min once per week for an additional 4 weeks. At 5 min after the intense exercise, lactate concentrations and lactate dehydrogenase (LDH) activity in the serum and the gastrocnemius muscle were higher in the control group, whereas M-EPPH and H-EPPH treatments suppressed the increase better than in the positive-control (p < 0.05). Intense exercise decreased glycogen content in the liver and gastrocnemius muscle, and M-EPPH and H-EPPH inhibited the decrement (p < 0.05). Moreover, lipid peroxide contents in the gastrocnemius muscle and liver were higher in the control group than in the M-EPPH, H-EPPH, positive-control, and no-exercise groups (p < 0.05). However, antioxidant enzyme activities such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were opposite to the lipid peroxide contents. Hypothalamic corticosterone and hippocampal mRNA expressions of tumor necrosis factor (TNF)-α and IL-1β were higher. However, hippocampal brain-derived neurotrophic factor (BDNF) mRNA expression and protein contents were lower in the control group than in the positive-control group. M-EPPH, H-EPPH, and positive-control suppressed the changes via activating hippocampal cAMP response element-binding protein phosphorylation, and H-EPPH showed better activity than in the positive-control (p < 0.05). In conclusion, EPPH (0.16−0.31 mL/kg BW) intake reduced exercise-induced physical and cognitive fatigue in rats and could potentially be developed as a therapeutic agent for relieving fatigue in humans.
Keywords: cognitive fatigue; exercise-induced fatigue; hypothalamus–pituitary–adrenaline axis; lactate; porcine placenta enzyme hydrolysates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Gentile D., Beeler D., Wang X.S., Ben-Ayre E., Zick S.M., Bao T., Carlson L.E., Ghelman R., Master V., Tripathy D., et al. Cancer-Related Fatigue Outcome Measures in Integrative Oncology: Evidence for Practice and Research Recommendations. Oncology. 2022;36:276–287. - PubMed
-
- Sapra A., Bhandari P. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Chronic Fatigue Syndrome. - PubMed
LinkOut - more resources
Full Text Sources

