The Contribution of the Locus Coeruleus-Noradrenaline System Degeneration during the Progression of Alzheimer's Disease
- PMID: 36552331
- PMCID: PMC9775634
- DOI: 10.3390/biology11121822
The Contribution of the Locus Coeruleus-Noradrenaline System Degeneration during the Progression of Alzheimer's Disease
Abstract
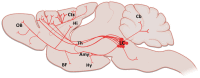
Alzheimer's disease (AD), which is characterized by extracellular accumulation of amyloid-beta peptide and intracellular aggregation of hyperphosphorylated tau, is the most common form of dementia. Memory loss, cognitive decline and disorientation are the ultimate consequences of neuronal death, synapse loss and neuroinflammation in AD. In general, there are many brain regions affected but neuronal loss in the locus coeruleus (LC) is one of the earliest indicators of neurodegeneration in AD. Since the LC is the main source of noradrenaline (NA) in the brain, degeneration of the LC in AD leads to decreased NA levels, causing increased neuroinflammation, enhanced amyloid and tau burden, decreased phagocytosis and impairment in cognition and long-term synaptic plasticity. In this review, we summarized current findings on the locus coeruleus-noradrenaline system and consequences of its dysfunction which is now recognized as an important contributor to AD progression.
Keywords: Alzheimer’s disease; cognition; locus coeruleus; neurodegeneration; neuroinflammation; noradrenaline; norepinephrine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Alzheimer Reports. [(accessed on 20 December 2019)]. Available online: https://www.alz.co.uk/research/world-report.
-
- Yankner B.A., Mesulam M.-M. β-Amyloid and the Pathogenesis of Alzheimer’s Disease. N. Engl. J. Med. 1991;325:1849–1857. - PubMed
-
- Roher A.E., Esh C.L., Kokjohn T.A., Castaño E.M., Van Vickle G.D., Kalback W.M., Patton R.L., Luehrs D.C., Daugs I.D., Kuo Y., et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimer’s Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

