The Complete Mitogenome of Toxocara vitulorum: Novel In-Sights into the Phylogenetics in Toxocaridae
- PMID: 36552470
- PMCID: PMC9774135
- DOI: 10.3390/ani12243546
The Complete Mitogenome of Toxocara vitulorum: Novel In-Sights into the Phylogenetics in Toxocaridae
Abstract
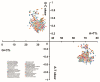
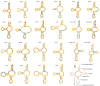
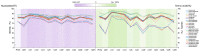
Toxocara vitulorum (Ascaridida: Nematoda) is one of the most common intestinal nematodes of cattle and buffalos and, therefore, represents a serious threat to their populations worldwide. Despite its significance in veterinary health the epidemiology, population genetics, and molecular ecology of this nematode remain poorly understood. The mitogenome can yield a foundation for studying these areas and assist in the surveillance and control of T. vitulorum. Herein, the first whole mitogenome of T. vitulorum was sequenced utilizing Illumina technology and characterized with bioinformatic pipeline analyses. The entire genome of T. vitulorum was 15,045 bp in length and contained 12 protein-coding genes (PCGs), 22 transfer RNAs (tRNAs), and two ribosomal RNAs (rRNAs). The gene arrangement (GA) of T. vitulorum was similar to those of other Toxocara species under GA3. The whole genome showed significant levels of AT and GC skew. Comparative mitogenomics including sequence identities, Ka/Ks, and sliding window analysis, indicated a purifying selection of 12 PCGs with cox1 and nad6 having the lowest and highest evolutionary rate, respectively. Whole amino acid sequence-based phylogenetic analysis supported a novel sister-species relationship of T. vitulorum with the congeneric species Toxocara canis, Toxocara cati, and Toxocara malaysiensis in the family Toxocaridae. Further, 12 (PCGs) single gene-based phylogenies suggested that nad4 and nad6 genes shared same topological trees with that of the whole genome, suggesting that these genes were suitable as novel genetic markers for phylogenetic and evolutionary studies of Ascaridida species. This complete mitogenome of T. vitulorum refined phylogenetic relationships in Toxocaridae and provided the resource of markers for population genetics, systematics, and epidemiology of this bovine nematode.
Keywords: Toxocara; mitochondrial genome; phylogenetic relationships; roundworms.
Conflict of interest statement
The funders had no role in the design of the study.
Figures



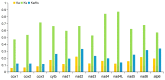

Similar articles
-
Characterisation of the mitochondrial genome and phylogenetic analysis of Toxocara apodemi (Nematoda: Ascarididae).J Helminthol. 2024 Apr 15;98:e33. doi: 10.1017/S0022149X24000221. J Helminthol. 2024. PMID: 38618902
-
Toxocara vitulorum (Ascaridida: Nematoda): mitochondrial gene content, arrangement and composition compared with other Toxocara species.Mol Biochem Parasitol. 2009 Jul;166(1):89-92. doi: 10.1016/j.molbiopara.2009.02.012. Epub 2009 Mar 5. Mol Biochem Parasitol. 2009. PMID: 19428679
-
The complete mitochondrial genomes for three Toxocara species of human and animal health significance.BMC Genomics. 2008 May 16;9:224. doi: 10.1186/1471-2164-9-224. BMC Genomics. 2008. PMID: 18482460 Free PMC article.
-
Toxocara canis and Toxocara vitulorum: molecular characterization, discrimination, and phylogenetic analysis based on mitochondrial (ATP synthase subunit 6 and 12S) and nuclear ribosomal (ITS-2 and 28S) genes.Parasitol Res. 2009 Jun;104(6):1425-30. doi: 10.1007/s00436-009-1345-9. Epub 2009 Feb 17. Parasitol Res. 2009. PMID: 19221796
-
Advances in molecular identification, taxonomy, genetic variation and diagnosis of Toxocara spp.Infect Genet Evol. 2012 Oct;12(7):1344-8. doi: 10.1016/j.meegid.2012.04.019. Epub 2012 Apr 28. Infect Genet Evol. 2012. PMID: 22569289 Review.
Cited by
-
Mitogenomic phylogenies suggest the resurrection of the subfamily Porrocaecinae and provide insights into the systematics of the superfamily Ascaridoidea (Nematoda: Ascaridomorpha), with the description of a new species of Porrocaecum.Parasit Vectors. 2023 Aug 10;16(1):275. doi: 10.1186/s13071-023-05889-9. Parasit Vectors. 2023. PMID: 37563590 Free PMC article.
-
Targeted pre-partum strategies to suppress Toxocara vitulorum hypobiotic larvae: Reducing transmission to calves and genotypic insights into buffalo infections.Vet World. 2025 Feb;18(2):329-340. doi: 10.14202/vetworld.2025.329-340. Epub 2025 Feb 13. Vet World. 2025. PMID: 40182831 Free PMC article.
-
Integrated evidence reveals a new subspecies of the genus Seuratascaris (Nematoda: Ascaridomorpha), with characterization of the complete mitochondrial genome.Parasite. 2025;32:14. doi: 10.1051/parasite/2025008. Epub 2025 Feb 24. Parasite. 2025. PMID: 39996964 Free PMC article.
-
Comparative mitogenomics reveals evolutionary drivers of Strongyloidea nematodes dwelling in gastrointestinal tract.BMC Genomics. 2025 Sep 1;26(1):793. doi: 10.1186/s12864-025-11980-5. BMC Genomics. 2025. PMID: 40890580 Free PMC article.
-
Year after year: Recurrent Toxocara vitulorum infections in American bison (Bison bison) calves in a zoo.Int J Parasitol Parasites Wildl. 2024 Nov 8;25:101018. doi: 10.1016/j.ijppaw.2024.101018. eCollection 2024 Dec. Int J Parasitol Parasites Wildl. 2024. PMID: 39659985 Free PMC article.
References
-
- Dorny P., Devleesschauwer B., Stoliaroff V., Sothy M., Chea R., Chea B., Sourloing H., Samuth S., Kong S., Nguong K., et al. Prevalence and Associated Risk Factors of Toxocara vitulorum Infections in Buffalo and Cattle Calves in Three Provinces of Central Cambodia. Korean J. Parasitol. 2015;53:197–200. doi: 10.3347/kjp.2015.53.2.197. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

