Thiocyanate Reduces Motor Impairment in the hMPO-A53T PD Mouse Model While Reducing MPO-Oxidation of Alpha Synuclein in Enlarged LYVE1/AQP4 Positive Periventricular Glymphatic Vessels
- PMID: 36552550
- PMCID: PMC9774557
- DOI: 10.3390/antiox11122342
Thiocyanate Reduces Motor Impairment in the hMPO-A53T PD Mouse Model While Reducing MPO-Oxidation of Alpha Synuclein in Enlarged LYVE1/AQP4 Positive Periventricular Glymphatic Vessels
Abstract
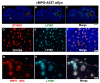
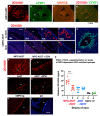
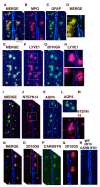
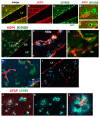
Parkinson's disease (PD) is due to the oxidation of alpha synuclein (αSyn) contributing to motor impairment. We developed a transgenic mouse model of PD that overexpresses the mutated human αSyn gene (A53T) crossed to a mouse expressing the human MPO gene. This model exhibits increased oxidation and chlorination of αSyn leading to greater motor impairment. In the current study, the hMPO-A53T mice were treated with thiocyanate (SCN-) which is a favored substrate of MPO as compared to chlorine. We show that hMPO-A53T mice treated with SCN- have less chlorination in the brain and show an improvement in motor skills compared to the nontreated hMPO-A53T mice. Interestingly, in the hMPO-A53T mice we found a possible link between MPO-related disease and the glymphatic system which clears waste including αSyn from the brain. The untreated hMPO-A53T mice exhibited an increase in the size of periventricular glymphatic vessels expressing the glymphatic marker LYVE1 and aquaporin 4 (AQP4). These vessels also exhibited an increase in MPO and HOCl-modified epitopes in the glymphatic vessels correlating with loss of ependymal cells lining the ventricles. These findings suggest that MPO may significantly promote the impairment of the glymphatic waste removal system thus contributing to neurodegeneration in PD. Moreover, the inhibition of MPO chlorination/oxidation by SCN- may provide a potential therapeutic approach to this disease.
Keywords: Parkinson’s disease; alpha synuclein; aquaporin 4; carbamylation; endothelial hyaluronan receptor 1; glial fibrillary acidic protein; glymphatics; hypochlorous acid; myeloperoxidase; nitration; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Human myeloperoxidase (hMPO) is expressed in neurons in the substantia nigra in Parkinson's disease and in the hMPO-α-synuclein-A53T mouse model, correlating with increased nitration and aggregation of α-synuclein and exacerbation of motor impairment.Free Radic Biol Med. 2019 Sep;141:115-140. doi: 10.1016/j.freeradbiomed.2019.05.033. Epub 2019 Jun 6. Free Radic Biol Med. 2019. PMID: 31175983 Free PMC article.
-
Blocking meningeal lymphatic drainage aggravates Parkinson's disease-like pathology in mice overexpressing mutated α-synuclein.Transl Neurodegener. 2019 Mar 1;8:7. doi: 10.1186/s40035-019-0147-y. eCollection 2019. Transl Neurodegener. 2019. PMID: 30867902 Free PMC article.
-
Interaction Between the Glymphatic System and α-Synuclein in Parkinson's Disease.Mol Neurobiol. 2023 Apr;60(4):2209-2222. doi: 10.1007/s12035-023-03212-2. Epub 2023 Jan 13. Mol Neurobiol. 2023. PMID: 36637746
-
Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson's Disease.Int J Mol Sci. 2022 Oct 26;23(21):12928. doi: 10.3390/ijms232112928. Int J Mol Sci. 2022. PMID: 36361716 Free PMC article. Review.
-
Gangliosides, α-Synuclein, and Parkinson's Disease.Prog Mol Biol Transl Sci. 2018;156:435-454. doi: 10.1016/bs.pmbts.2017.12.009. Epub 2018 Feb 24. Prog Mol Biol Transl Sci. 2018. PMID: 29747823 Review.
Cited by
-
Association between serum myeloperoxidase enzyme activity and Parkinson's disease status.NPJ Parkinsons Dis. 2025 Apr 26;11(1):94. doi: 10.1038/s41531-025-00941-0. NPJ Parkinsons Dis. 2025. PMID: 40287421 Free PMC article.
-
Nrf2 and Ferroptosis: Exploring Translational Avenues for Therapeutic Approaches to Neurological Diseases.Curr Drug Targets. 2025;26(1):33-58. doi: 10.2174/0113894501320839240918110656. Curr Drug Targets. 2025. PMID: 39350404 Review.
References
-
- Perry T.L., Yong V.W. Idiopathic Parkinson’s disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. [(accessed on 21 October 2022)];Neurosci. Lett. 1986 67:269–274. doi: 10.1016/0304-3940(86)90320-4. Available online: https://www.ncbi.nlm.nih.gov/pubmed/3737015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

