The Sulfide-Responsive SqrR/BigR Homologous Regulator YgaV of Escherichia coli Controls Expression of Anaerobic Respiratory Genes and Antibiotic Tolerance
- PMID: 36552568
- PMCID: PMC9774250
- DOI: 10.3390/antiox11122359
The Sulfide-Responsive SqrR/BigR Homologous Regulator YgaV of Escherichia coli Controls Expression of Anaerobic Respiratory Genes and Antibiotic Tolerance
Abstract
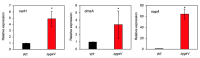
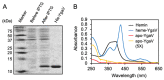
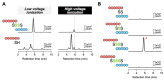
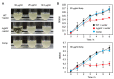
Compositions and activities of bacterial flora in the gastrointestinal tract significantly influence the metabolism, health, and disease of host humans and animals. These enteric bacteria can switch between aerobic and anaerobic growth if oxygen tension becomes limited. Interestingly, the switching mechanism is important for preventing reactive oxygen species (ROS) production and antibiotic tolerance. Studies have also shown that intracellular and extracellular sulfide molecules are involved in this switching control, although the mechanism is not fully clarified. Here, we found that YgaV, a sulfide-responsive transcription factor SqrR/BigR homolog, responded to sulfide compounds in vivo and in vitro to control anaerobic respiratory gene expression. YgaV also responded to H2O2 scavenging in the enteric bacterium Escherichia coli. Although the wild-type (WT) showed increased antibiotic tolerance under H2S-atmospheric conditions, the ygaV mutant did not show such a phenotype. Additionally, antibiotic sensitivity was higher in the mutant than in the WT of both types in the presence and absence of exogenous H2S. These results, therefore, indicated that YgaV-dependent transcriptional regulation was responsible for maintaining redox homeostasis, ROS scavenging, and antibiotic tolerance.
Keywords: Escherichia coli; SqrR; YgaV; antibiotics; hydrogen sulfide; reactive sulfur species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Sulfide-Responsive Transcription Control in Escherichia coli.Microorganisms. 2025 Feb 5;13(2):344. doi: 10.3390/microorganisms13020344. Microorganisms. 2025. PMID: 40005711 Free PMC article.
-
Heme bound to the bacterial transcription factor SqrR/YgaV catalyzes oxygen-dependent conversion of hydrogen sulfide to polysulfide for regulated gene expression.Redox Biol. 2025 Jul 31;86:103801. doi: 10.1016/j.redox.2025.103801. Online ahead of print. Redox Biol. 2025. PMID: 40763654 Free PMC article.
-
Increased intracellular H2S levels enhance iron uptake in Escherichia coli.mBio. 2024 Oct 16;15(10):e0199124. doi: 10.1128/mbio.01991-24. Epub 2024 Sep 26. mBio. 2024. PMID: 39324809 Free PMC article.
-
Cellular and molecular physiology of Escherichia coli in the adaptation to aerobic environments.J Biochem. 1996 Dec;120(6):1055-63. doi: 10.1093/oxfordjournals.jbchem.a021519. J Biochem. 1996. PMID: 9010748 Review.
-
Sulfur Cycling and the Intestinal Microbiome.Dig Dis Sci. 2017 Sep;62(9):2241-2257. doi: 10.1007/s10620-017-4689-5. Epub 2017 Aug 1. Dig Dis Sci. 2017. PMID: 28766244 Review.
Cited by
-
In the Alphaproteobacterium Hyphomicrobium denitrificans SoxR Serves a Sulfane Sulfur-Responsive Repressor of Sulfur Oxidation.Antioxidants (Basel). 2023 Aug 16;12(8):1620. doi: 10.3390/antiox12081620. Antioxidants (Basel). 2023. PMID: 37627615 Free PMC article.
-
Sensing and regulation of reactive sulfur species (RSS) in bacteria.Curr Opin Chem Biol. 2023 Oct;76:102358. doi: 10.1016/j.cbpa.2023.102358. Epub 2023 Jul 1. Curr Opin Chem Biol. 2023. PMID: 37399745 Free PMC article. Review.
-
Sulfide-Responsive Transcription Control in Escherichia coli.Microorganisms. 2025 Feb 5;13(2):344. doi: 10.3390/microorganisms13020344. Microorganisms. 2025. PMID: 40005711 Free PMC article.
-
Increased intracellular persulfide levels attenuate HlyU-mediated hemolysin transcriptional activation in Vibrio cholerae.bioRxiv [Preprint]. 2023 Mar 13:2023.03.13.532278. doi: 10.1101/2023.03.13.532278. bioRxiv. 2023. Update in: J Biol Chem. 2023 Sep;299(9):105147. doi: 10.1016/j.jbc.2023.105147. PMID: 36993174 Free PMC article. Updated. Preprint.
-
Bacterial Metallostasis: Metal Sensing, Metalloproteome Remodeling, and Metal Trafficking.Chem Rev. 2024 Dec 25;124(24):13574-13659. doi: 10.1021/acs.chemrev.4c00264. Epub 2024 Dec 10. Chem Rev. 2024. PMID: 39658019 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

