Size-Controllable Prussian Blue Nanoparticles Using Pluronic Series for Improved Antioxidant Activity and Anti-Inflammatory Efficacy
- PMID: 36552600
- PMCID: PMC9774457
- DOI: 10.3390/antiox11122392
Size-Controllable Prussian Blue Nanoparticles Using Pluronic Series for Improved Antioxidant Activity and Anti-Inflammatory Efficacy
Abstract
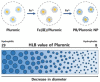
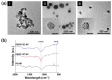
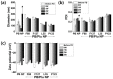
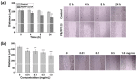
Prussian blue (PB) is a metal cluster nanoparticle (NP) of cyanide-bridged iron(II)-iron(III) and exhibits a characteristic blue color. Its peroxidase-, catalase-, and superoxide-dismutase-like activities effectively remove excess reactive oxygen species that induce inflammation and tumorigenesis. However, the dispersion of PB NPs is not sufficiently stable for their application in the biomedical field. In this study, we developed Pluronic-stabilized Prussian blue nanoparticles (PB/Plu NPs) using a series of Pluronic triblock copolymers as a template material for PB NPs. Considering the hydrophilic-lipophilic balance (HLB) values of the Pluronic series, including F68, F127, L35, P123, and L81, the diameters of the PB/Plu NPs decreased from 294 to 112 nm with decreasing HLB values. The smallest PB NP stabilized with Pluronic P123 (PB/PP123 NP) showed the strongest antioxidant and anti-inflammatory activities and wound-healing efficacy because of its large surface area. These results indicated that the spatial distribution of PB NPs in the micelles of Pluronic greatly improved the stability and reactive oxygen species scavenging activity of these NPs. Therefore, PB/Plu NPs using U.S.-FDA-approved Pluronic polymers show potential as biocompatible materials for various biomedical applications, including the treatment of inflammatory diseases in the clinic.
Keywords: Pluronic; Prussian blue; anti-inflammation; antioxidant; reactive oxygen species; wound healing.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

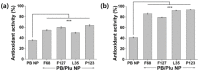
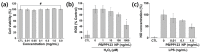
References
-
- Bandyopadhyay U., Das D., Banerjee R.K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999;77:658–666.
-
- Halliwell B. Free radicals and other reactive species in disease. eLS. 2015;1:1–9. doi: 10.1002/9780470015902.a0002269.pub3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

