Mangosteen Pericarp Extract Supplementation Boosts Antioxidant Status via Rebuilding Gut Microbiota to Attenuate Motor Deficit in 6-OHDA-Induced Parkinson's Disease
- PMID: 36552604
- PMCID: PMC9774421
- DOI: 10.3390/antiox11122396
Mangosteen Pericarp Extract Supplementation Boosts Antioxidant Status via Rebuilding Gut Microbiota to Attenuate Motor Deficit in 6-OHDA-Induced Parkinson's Disease
Abstract
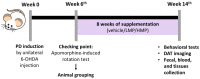
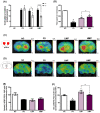
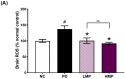
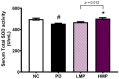
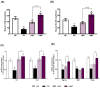
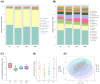
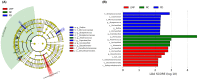
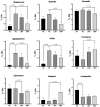
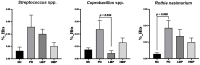
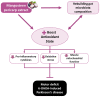
Oxidative stress and gut dysbiosis have been known to precede Parkinson's disease (PD). An antioxidant-rich product, mangosteen pericarp (MP), has the ability to counterbalance excessive free radicals and the imbalanced gut microbiota composition, suggesting the MP's capacity to delay PD progression. In this study, we explored the effects of two doses of MP extract in a unilateral 6-hydroxydopamine (6-OHDA)-induced PD rat model. We revealed that the 8-week supplementation of a low dose (LMP) and a high dose of the MP extract (HMP) improved motor function, as observed in decreased contralateral rotation, improved time spent on rod, and higher dopamine binding transporter (DAT) in the substantia nigra pars compacta (SNc). The MP extract, especially the HMP, also increased antioxidant-related gene expressions, restored muscle mitochondrial function, and remodeled fecal microbiota composition, which were followed by reduced reactive oxygen species levels in brain and inflammation in plasma. Importantly, bacterial genera Sutterella, Rothia, and Aggregatibacter, which were negatively correlated with antioxidant gene expressions, decreased in the HMP group. It is imperative to note that in addition to directly acting as an antioxidant to reduce excessive free radicals, MP extract might also increase antioxidant state by rebuilding gut microbiota, thereby enhanced anti-inflammatory capacity and restored mitochondrial function to attenuate motor deficit in 6-OHDA-induced PD-like condition. All in all, MP extract is a potential candidate for auxiliary therapy for PD.
Keywords: 6-hydroxydopamine; Parkinson’s disease; antioxidant; fecal microbiota composition; mangosteen pericarp.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson's Disease Rats.Antioxidants (Basel). 2021 Nov 17;10(11):1823. doi: 10.3390/antiox10111823. Antioxidants (Basel). 2021. PMID: 34829694 Free PMC article.
-
Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson's disease.Exp Neurol. 2020 Mar;325:113159. doi: 10.1016/j.expneurol.2019.113159. Epub 2019 Dec 13. Exp Neurol. 2020. PMID: 31843492
-
Dysbiosis of gut microbiota inhibits NMNAT2 to promote neurobehavioral deficits and oxidative stress response in the 6-OHDA-lesioned rat model of Parkinson's disease.J Neuroinflammation. 2023 May 19;20(1):117. doi: 10.1186/s12974-023-02782-1. J Neuroinflammation. 2023. PMID: 37208728 Free PMC article.
-
Chronic stress-induced gut dysfunction exacerbates Parkinson's disease phenotype and pathology in a rotenone-induced mouse model of Parkinson's disease.Neurobiol Dis. 2020 Feb;135:104352. doi: 10.1016/j.nbd.2018.12.012. Epub 2018 Dec 21. Neurobiol Dis. 2020. PMID: 30579705 Review.
-
Mucuna pruriens in Parkinson's and in some other diseases: recent advancement and future prospective.3 Biotech. 2020 Dec;10(12):522. doi: 10.1007/s13205-020-02532-7. Epub 2020 Nov 10. 3 Biotech. 2020. PMID: 33194526 Free PMC article. Review.
Cited by
-
Oral microbiome dysbiosis may be associated with intra cranial aneurysms.BMC Oral Health. 2024 Oct 16;24(1):1235. doi: 10.1186/s12903-024-05015-w. BMC Oral Health. 2024. PMID: 39415150 Free PMC article.
-
The Associations Among Gut Microbiota, Branched Chain Amino Acids, and Parkinson's Disease: Mendelian Randomization Study.J Parkinsons Dis. 2024;14(6):1129-1138. doi: 10.3233/JPD-240244. J Parkinsons Dis. 2024. PMID: 39177611 Free PMC article.
-
Exploring the role of gut microbiota in Parkinson's disease: insights from fecal microbiota transplantation.Front Neurosci. 2025 Jun 13;19:1574512. doi: 10.3389/fnins.2025.1574512. eCollection 2025. Front Neurosci. 2025. PMID: 40584885 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

