The Alleviation of Dextran Sulfate Sodium (DSS)-Induced Colitis Correlate with the log P Values of Food-Derived Electrophilic Compounds
- PMID: 36552614
- PMCID: PMC9774124
- DOI: 10.3390/antiox11122406
The Alleviation of Dextran Sulfate Sodium (DSS)-Induced Colitis Correlate with the log P Values of Food-Derived Electrophilic Compounds
Abstract
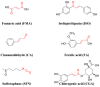
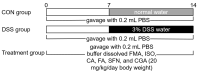
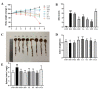
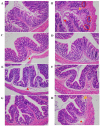
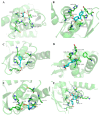
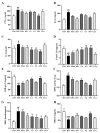
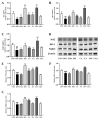
Food-derived electrophilic compounds (FECs) are small molecules with electrophilic groups with potential cytoprotective effects. This study investigated the differential effects of six prevalent FECs on colitis in dextran sodium sulfate (DSS)-induced mice and the underlying relationship with molecular characteristics. Fumaric acid (FMA), isoliquiritigenin (ISO), cinnamaldehyde (CA), ferulic acid (FA), sulforaphane (SFN), and chlorogenic acid (CGA) exhibited varying improvements in colitis on clinical signs, colonic histopathology, inflammatory and oxidative indicators, and Nrf2 pathway in a sequence of SFN, ISO > FA, CA > FMA, CGA. Representative molecular characteristics of the “penetration-affinity−covalent binding” procedure, logP value, Keap1 affinity energy, and electrophilic index of FECs were theoretically calculated, among which logP value revealed a strong correlation with colitis improvements, which was related to the expression of Nrf2 and its downstream proteins. Above all, SFN and ISO possessed high logP values and effectively improving DSS-induced colitis by activating the Keap1−Nrf2 pathway to alleviate oxidative stress and inflammatory responses.
Keywords: colitis; electrophilic compound; logP; molecular characteristic.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
-
- Keshteli A.H., van den Brand F.F., Madsen K.L., Mandal R., Valcheva R., Kroeker K.I., Han B., Bell R.C., Cole J., Hoevers T., et al. Dietary and metabolomic determinants of relapse in ulcerative colitis patients: A pilot prospective cohort study. World J. Gastroenterol. 2017;23:3890–3899. doi: 10.3748/wjg.v23.i21.3890. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

