Nicotine and Cotinine Induce Neutrophil Extracellular Trap Formation-Potential Risk for Impaired Wound Healing in Smokers
- PMID: 36552632
- PMCID: PMC9774423
- DOI: 10.3390/antiox11122424
Nicotine and Cotinine Induce Neutrophil Extracellular Trap Formation-Potential Risk for Impaired Wound Healing in Smokers
Abstract
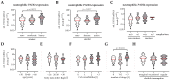
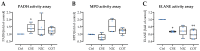
Smoking undoubtedly affects human health. Investigating 2318 representative patients at a level 1 trauma center identified delayed wound healing, tissue infections, and/or sepsis as main complications in smokers following trauma and orthopedic surgery. Therefore, smoking cessation is strongly advised to improve the clinical outcome in these patients, although smoking cessation often fails despite nicotine replacement therapy raising the need for specific interventions that may reduce the complication rate. However, the underlying mechanisms are still unknown. In diabetics, delayed wound healing and infections/sepsis are associated with increased neutrophilic PADI4 expression and formation of neutrophil extracellular traps (NETs). The aim was to investigate if similar mechanisms hold for smokers. Indeed, our results show higher PADI4 expression in active and heavy smokers than non-smokers, which is associated with an increased complication rate. However, in vitro stimulation of neutrophils with cigarette smoke extract (CSE) only moderately induced NET formation despite accumulation of reactive oxygen species (ROS). Physiological levels of nicotine and its main metabolite cotinine more effectively induced NET formation, although they did not actively induce the formation of ROS, but interfered with the activity of enzymes involved in anti-oxidative defense and NET formation. In summary, we propose increased formation of NETs as possible triggers for delayed wound healing, tissue infections, and/or sepsis in smokers after a major trauma and orthopedic surgery. Smoking cessation might reduce this effect. However, our data show that smoking cessation supported by nicotine replacement therapy should be carefully considered as nicotine and its metabolite cotinine effectively induced NET formation in vitro, even without active formation of ROS.
Keywords: cotinine; neutrophil extracellular traps; nicotine; reactive oxygen species; smoking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Cigarette smoke modifies neutrophil chemotaxis, neutrophil extracellular trap formation and inflammatory response-related gene expression.J Periodontal Res. 2018 Aug;53(4):525-535. doi: 10.1111/jre.12542. Epub 2018 Mar 25. J Periodontal Res. 2018. PMID: 29574730
-
Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke.Oxid Med Cell Longev. 2018 Nov 6;2018:3172480. doi: 10.1155/2018/3172480. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30533170 Free PMC article.
-
Molecular and functional changes in neutrophilic granulocytes induced by nicotine: a systematic review and critical evaluation.Front Immunol. 2023 Nov 23;14:1281685. doi: 10.3389/fimmu.2023.1281685. eCollection 2023. Front Immunol. 2023. PMID: 38077313 Free PMC article.
-
Application of serum nicotine and plasma cotinine concentrations to assessment of nicotine replacement in light, moderate, and heavy smokers undergoing transdermal therapy.J Clin Pharmacol. 1998 Jun;38(6):502-9. doi: 10.1002/j.1552-4604.1998.tb05787.x. J Clin Pharmacol. 1998. PMID: 9650539 Clinical Trial.
-
A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations.Clin Ther. 2008 May;30(5):800-12. doi: 10.1016/j.clinthera.2008.05.010. Clin Ther. 2008. PMID: 18555928 Review.
Cited by
-
In Vitro Modeling of Diurnal Changes in Bone Metabolism.Int J Mol Sci. 2025 Aug 8;26(16):7699. doi: 10.3390/ijms26167699. Int J Mol Sci. 2025. PMID: 40869020 Free PMC article.
-
Mendelian randomization analysis reveals causal associations of serum metabolites with sepsis and 28-day mortality.Sci Rep. 2024 May 21;14(1):11551. doi: 10.1038/s41598-024-58160-1. Sci Rep. 2024. PMID: 38773119 Free PMC article.
-
Long-Term Cigarette Smoke Exposure Promotes Neutrophil Ferroptosis Resistance, Inducing Neutrophil Extracellular Trap Formation and Driving Glucocorticoid Resistance in Chronic Obstructive Pulmonary Disease.Research (Wash D C). 2025 Jul 15;8:0751. doi: 10.34133/research.0751. eCollection 2025. Research (Wash D C). 2025. PMID: 40666830 Free PMC article.
-
[Effect of Smoking on Cancer Surgery Outcomes and Recommendations for Perioperative Smoking Cessation Intervention].Sichuan Da Xue Xue Bao Yi Xue Ban. 2023 Nov 20;54(6):1312-1316. doi: 10.12182/20231160605. Sichuan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 38162073 Free PMC article. Chinese.
-
Cancer treatments as paradoxical catalysts of tumor awakening in the lung.Cancer Metastasis Rev. 2024 Dec;43(4):1165-1183. doi: 10.1007/s10555-024-10196-5. Epub 2024 Jul 4. Cancer Metastasis Rev. 2024. PMID: 38963567 Free PMC article. Review.
References
-
- Ehnert S., Aspera-Werz R.H., Ihle C., Trost M., Zirn B., Flesch I., Schroter S., Relja B., Nussler A.K. Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. J. Clin. Med. 2019;8:406. doi: 10.3390/jcm8030406. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

