Oxidative Regulation of Vascular Cav1.2 Channels Triggers Vascular Dysfunction in Hypertension-Related Disorders
- PMID: 36552639
- PMCID: PMC9774363
- DOI: 10.3390/antiox11122432
Oxidative Regulation of Vascular Cav1.2 Channels Triggers Vascular Dysfunction in Hypertension-Related Disorders
Abstract
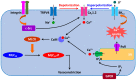
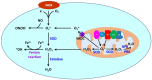
Blood pressure is determined by cardiac output and peripheral vascular resistance. The L-type voltage-gated Ca2+ (Cav1.2) channel in small arteries and arterioles plays an essential role in regulating Ca2+ influx, vascular resistance, and blood pressure. Hypertension and preeclampsia are characterized by high blood pressure. In addition, diabetes has a high prevalence of hypertension. The etiology of these disorders remains elusive, involving the complex interplay of environmental and genetic factors. Common to these disorders are oxidative stress and vascular dysfunction. Reactive oxygen species (ROS) derived from NADPH oxidases (NOXs) and mitochondria are primary sources of vascular oxidative stress, whereas dysfunction of the Cav1.2 channel confers increased vascular resistance in hypertension. This review will discuss the importance of ROS derived from NOXs and mitochondria in regulating vascular Cav1.2 and potential roles of ROS-mediated Cav1.2 dysfunction in aberrant vascular function in hypertension, diabetes, and preeclampsia.
Keywords: Cav1.2; gestational diabetes; hypertension; myogenic tone; preeclampsia; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Aberrant Splicing Induced by Dysregulated Rbfox2 Produces Enhanced Function of CaV1.2 Calcium Channel and Vascular Myogenic Tone in Hypertension.Hypertension. 2017 Dec;70(6):1183-1192. doi: 10.1161/HYPERTENSIONAHA.117.09301. Epub 2017 Oct 9. Hypertension. 2017. PMID: 28993448
-
Diminished Rbfox1 increases vascular constriction by dynamically regulating alternative splicing of CaV1.2 calcium channel in hypertension.Clin Sci (Lond). 2022 Jun 17;136(11):803-817. doi: 10.1042/CS20220226. Clin Sci (Lond). 2022. PMID: 35543237
-
Glucose-6-phosphate dehydrogenase increases Ca2+ currents by interacting with Cav1.2 and reducing intrinsic inactivation of the L-type calcium channel.Am J Physiol Heart Circ Physiol. 2020 Jul 1;319(1):H144-H158. doi: 10.1152/ajpheart.00727.2019. Epub 2020 May 22. Am J Physiol Heart Circ Physiol. 2020. PMID: 32442021 Free PMC article.
-
Ion channels and the regulation of myogenic tone in peripheral arterioles.Curr Top Membr. 2020;85:19-58. doi: 10.1016/bs.ctm.2020.01.002. Epub 2020 Feb 25. Curr Top Membr. 2020. PMID: 32402640 Free PMC article. Review.
-
Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles.Compr Physiol. 2017 Mar 16;7(2):485-581. doi: 10.1002/cphy.c160011. Compr Physiol. 2017. PMID: 28333380 Free PMC article. Review.
Cited by
-
LRRC8A anion channels modulate vascular reactivity via association with myosin phosphatase rho interacting protein.FASEB J. 2023 Jul;37(7):e23028. doi: 10.1096/fj.202300561R. FASEB J. 2023. PMID: 37310356 Free PMC article.
-
NADPH oxidases: redox regulation of cell homeostasis and disease.Physiol Rev. 2025 Jul 1;105(3):1291-1428. doi: 10.1152/physrev.00034.2023. Epub 2025 Jan 15. Physiol Rev. 2025. PMID: 39814410 Free PMC article. Review.
-
Hypertension and cellular senescence.Biogerontology. 2023 Aug;24(4):457-478. doi: 10.1007/s10522-023-10031-4. Epub 2023 Apr 3. Biogerontology. 2023. PMID: 37010665 Review.
-
Ahf-Caltide, a Novel Polypeptide Derived from Calpastatin, Protects against Oxidative Stress Injury by Stabilizing the Expression of CaV1.2 Calcium Channel.Int J Mol Sci. 2023 Oct 29;24(21):15729. doi: 10.3390/ijms242115729. Int J Mol Sci. 2023. PMID: 37958713 Free PMC article.
-
SHP1 and its downstream p38/SP1/PI3K/YAP/Notch-1 signaling in trophoblast cells suppressed the progression of Preeclampsia via inhibiting proliferation of SMCs.Sci Rep. 2025 May 9;15(1):16205. doi: 10.1038/s41598-025-00164-6. Sci Rep. 2025. PMID: 40346122 Free PMC article.
References
-
- Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

