Protective Actions of α-Tocopherol on Cell Membrane Lipids of Paraquat-Stressed Human Astrocytes Using Microarray Technology, MALDI-MS and Lipidomic Analysis
- PMID: 36552648
- PMCID: PMC9774397
- DOI: 10.3390/antiox11122440
Protective Actions of α-Tocopherol on Cell Membrane Lipids of Paraquat-Stressed Human Astrocytes Using Microarray Technology, MALDI-MS and Lipidomic Analysis
Abstract
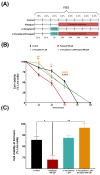
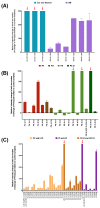
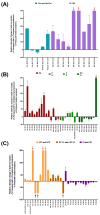
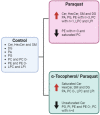
Cellular senescence is one of the main contributors to some neurodegenerative disorders. The early detection of senescent cells or their related effects is a key aspect in treating disease progression. In this functional deterioration, oxidative stress and lipid peroxidation play an important role. Endogenous antioxidant compounds, such as α-tocopherol (vitamin E), can mitigate these undesirable effects, particularly lipid peroxidation, by blocking the reaction between free radicals and unsaturated fatty acid. While the antioxidant actions of α-tocopherol have been studied in various systems, monitoring the specific effects on cell membrane lipids at scales compatible with large screenings has not yet been accomplished. Understanding the changes responsible for this protection against one of the consequences of senescence is therefore necessary. Thus, the goal of this study was to determinate the changes in the lipid environment of a Paraquat-treated human astrocytic cell line, as a cellular oxidative stress model, and the specific actions of the antioxidant, α-tocopherol, using cell membrane microarray technology, MALDI-MS and lipidomic analysis. The stress induced by Paraquat exposure significantly decreased cell viability and triggered membrane lipid changes, such as an increase in certain species of ceramides that are lipid mediators of apoptotic pathways. The pre-treatment of cells with α-tocopherol mitigated these effects, enhancing cell viability and modulating the lipid profile in Paraquat-treated astrocytes. These results demonstrate the lipid modulation effects of α-tocopherol against Paraquat-promoted oxidative stress and validate a novel analytical high-throughput method combining cell cultures, microarray technology, MALDI-MS and multivariate analysis to study antioxidant compounds against cellular senescence.
Keywords: astrocytes; human cell line; lipid peroxidation; membrane microarrays; oxidative stress.
Conflict of interest statement
All coauthors of IMG Pharma Biotech S.L. have no conflicts of interest in the present study for publication. E.A. and G.B.-G. are listed as inventors on patent (EP2048534A4). The remaining authors declare no competing interests.
Figures
Similar articles
-
Relative alpha-tocopherol deficiency in cultured cells: free radical-mediated lipid peroxidation, lipid oxidizability, and cellular polyunsaturated fatty acid content.Arch Biochem Biophys. 1995 May 10;319(1):102-9. doi: 10.1006/abbi.1995.1271. Arch Biochem Biophys. 1995. PMID: 7771773
-
How lipid unsaturation, peroxyl radical partitioning, and chromanol lipophilic tail affect the antioxidant activity of α-tocopherol: direct visualization via high-throughput fluorescence studies conducted with fluorogenic α-tocopherol analogues.J Am Chem Soc. 2012 Jun 20;134(24):10102-13. doi: 10.1021/ja301680m. Epub 2012 Jun 6. J Am Chem Soc. 2012. PMID: 22568598
-
Alpha-tocopherol protects against oxidative damage to lipids of the rod outer segments of the equine retina.Vet J. 2009 Dec;182(3):463-8. doi: 10.1016/j.tvjl.2008.08.008. Epub 2008 Oct 1. Vet J. 2009. PMID: 18829353
-
Interactions between α-tocopherol, polyunsaturated fatty acids, and lipoxygenases during embryogenesis.Free Radic Biol Med. 2014 Jan;66:13-9. doi: 10.1016/j.freeradbiomed.2013.07.039. Epub 2013 Aug 3. Free Radic Biol Med. 2014. PMID: 23920314 Free PMC article. Review.
-
Vitamin E and its function in membranes.Prog Lipid Res. 1999 Jul;38(4):309-36. doi: 10.1016/s0163-7827(99)00008-9. Prog Lipid Res. 1999. PMID: 10793887 Review.
Cited by
-
Microarray-Based Methodology for Lipid Profiling, Enzymatic Activity, And Binding Assays in Printed Lipid Raft Membranes from Astrocytes and Neurons.Anal Chem. 2025 Jan 14;97(1):86-95. doi: 10.1021/acs.analchem.4c02421. Epub 2024 Dec 24. Anal Chem. 2025. PMID: 39718364 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

