Maqui Berry and Ginseng Extracts Reduce Cigarette Smoke-Induced Cell Injury in a 3D Bone Co-Culture Model
- PMID: 36552669
- PMCID: PMC9774157
- DOI: 10.3390/antiox11122460
Maqui Berry and Ginseng Extracts Reduce Cigarette Smoke-Induced Cell Injury in a 3D Bone Co-Culture Model
Abstract
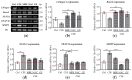
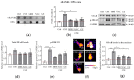
Cigarette smoking-induced oxidative stress has harmful effects on bone metabolism. Maqui berry extract (MBE) and ginseng extract (GE) are two naturally occurring antioxidants that have been shown to reduce oxidative stress. By using an osteoblast and osteoclast three-dimensional co-culture system, we investigated the effects of MBE and GE on bone cells exposed to cigarette smoke extract (CSE). The cell viability and function of the co-culture system were measured on day 14. Markers of bone cell differentiation and oxidative stress were evaluated at gene and protein levels on day 7. The results showed that exposure to CSE induced osteoporotic-like alterations in the co-culture system, while 1.5 µg/mL MBE and 50 µg/mL GE improved CSE-impaired osteoblast function and decreased CSE-induced osteoclast function. The molecular mechanism of MBE and GE in preventing CSE-induced bone cell damage is linked with the inhibition of the NF-κB signaling pathway and the activation of the Nrf2 signaling pathway. Therefore, MBE and GE can reduce CSE-induced detrimental effects on bone cells and, thus, prevent smoking-induced alterations in bone cell homeostasis. These two antioxidants are thus suitable supplements to support bone regeneration in smokers.
Keywords: cigarette smoke; co-culture system; ginseng; maqui berry; osteoblast; osteoclast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Maqui berry extract prevents cigarette smoke induced oxidative stress in human osteoblasts in vitro.EXCLI J. 2021 Feb 9;20:281-296. doi: 10.17179/excli2020-3244. eCollection 2021. EXCLI J. 2021. PMID: 33628164 Free PMC article.
-
Herbal Extracts of Ginseng and Maqui Berry Show Only Minimal Effects on an In Vitro Model of Early Fracture Repair of Smokers.Foods. 2023 Aug 4;12(15):2960. doi: 10.3390/foods12152960. Foods. 2023. PMID: 37569229 Free PMC article.
-
A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models.Antioxidants (Basel). 2019 Sep 10;8(9):386. doi: 10.3390/antiox8090386. Antioxidants (Basel). 2019. PMID: 31509995 Free PMC article.
-
Cigarette smoke increases pro-inflammatory markers and inhibits osteogenic differentiation in experimental exposure model.Acta Biomater. 2018 Aug;76:308-318. doi: 10.1016/j.actbio.2018.06.018. Epub 2018 Jun 12. Acta Biomater. 2018. PMID: 29902595
-
Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model.Int J Mol Sci. 2020 Dec 23;22(1):53. doi: 10.3390/ijms22010053. Int J Mol Sci. 2020. PMID: 33374546 Free PMC article.
Cited by
-
A Review of the Functional Characteristics and Applications of Aristotelia chilensis (Maqui Berry), in the Food Industry.Foods. 2024 Mar 9;13(6):838. doi: 10.3390/foods13060838. Foods. 2024. PMID: 38540828 Free PMC article. Review.
-
Impact of Particle Size and Sintering Temperature on Calcium Phosphate Gyroid Structure Scaffolds for Bone Tissue Engineering.J Funct Biomater. 2024 Nov 21;15(12):355. doi: 10.3390/jfb15120355. J Funct Biomater. 2024. PMID: 39728155 Free PMC article.
-
Aggregation of human osteoblasts unlocks self-reliant differentiation and constitutes a microenvironment for 3D-co-cultivation with other bone marrow cells.Sci Rep. 2024 May 6;14(1):10345. doi: 10.1038/s41598-024-60986-8. Sci Rep. 2024. PMID: 38710795 Free PMC article.
-
Establishment of a human 3D in vitro liver-bone model as a potential system for drug toxicity screening.Arch Toxicol. 2025 Jan;99(1):333-356. doi: 10.1007/s00204-024-03899-9. Epub 2024 Nov 6. Arch Toxicol. 2025. PMID: 39503877 Free PMC article.
-
In Vitro Modeling of Diurnal Changes in Bone Metabolism.Int J Mol Sci. 2025 Aug 8;26(16):7699. doi: 10.3390/ijms26167699. Int J Mol Sci. 2025. PMID: 40869020 Free PMC article.
References
-
- Heher P., Ganassi M., Weidinger A., Engquist E.N., Pruller J., Nguyen T.H., Tassin A., Declèves A.E., Mamchaoui K., Banerji C., et al. Interplay between mitochondrial reactive oxygen species, oxidative stress and hypoxic adaptation in facioscapulohumeral muscular dystrophy: Metabolic stress as potential therapeutic target. Redox Biol. 2022;51:102251. doi: 10.1016/j.redox.2022.102251. - DOI - PMC - PubMed
-
- Deng W., Ding Z., Wang Y., Zou B., Zheng J., Tan Y., Yang Q., Ke M., Chen Y., Wang S., et al. Dendrobine attenuates osteoclast differentiation through modulating ROS/NFATc1/MMP9 pathway and prevents inflammatory bone destruction. Phytomedicine. 2022;96:153838. doi: 10.1016/j.phymed.2021.153838. - DOI - PubMed
LinkOut - more resources
Full Text Sources

