Association between Heavy Metal Exposure and Parkinson's Disease: A Review of the Mechanisms Related to Oxidative Stress
- PMID: 36552676
- PMCID: PMC9774122
- DOI: 10.3390/antiox11122467
Association between Heavy Metal Exposure and Parkinson's Disease: A Review of the Mechanisms Related to Oxidative Stress
Abstract
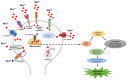
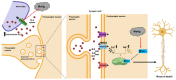
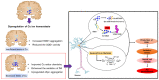
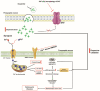
Parkinson's disease (PD) is a gradually progressing neurodegenerative condition that is marked by a loss of motor coordination along with non-motor features. Although the precise cause of PD has not been determined, the disease condition is mostly associated with the exposure to environmental toxins, such as metals, and their abnormal accumulation in the brain. Heavy metals, such as iron (Fe), mercury (Hg), manganese (Mn), copper (Cu), and lead (Pb), have been linked to PD and contribute to its progression. In addition, the interactions among the components of a metal mixture may result in synergistic toxicity. Numerous epidemiological studies have demonstrated a connection between PD and either single or mixed exposure to these heavy metals, which increase the prevalence of PD. Chronic exposure to heavy metals is related to the activation of proinflammatory cytokines resulting in neuronal loss through neuroinflammation. Similarly, metals disrupt redox homeostasis while inducing free radical production and decreasing antioxidant levels in the substantia nigra. Furthermore, these metals alter molecular processes and result in oxidative stress, DNA damage, mitochondrial dysfunction, and apoptosis, which can potentially trigger dopaminergic neurodegenerative disorders. This review focuses on the roles of Hg, Pb, Mn, Cu, and Fe in the development and progression of PD. Moreover, it explores the plausible roles of heavy metals in neurodegenerative mechanisms that facilitate the development of PD. A better understanding of the mechanisms underlying metal toxicities will enable the establishment of novel therapeutic approaches to prevent or cure PD.
Keywords: Parkinson’s disease; copper; heavy metals; iron; lead; manganese; mercury; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity.Chemosphere. 2022 Aug;301:134625. doi: 10.1016/j.chemosphere.2022.134625. Epub 2022 Apr 16. Chemosphere. 2022. PMID: 35439490 Review.
-
Metals associated neurodegeneration in Parkinson's disease: Insight to physiological, pathological mechanisms and management.Neurosci Lett. 2021 May 14;753:135873. doi: 10.1016/j.neulet.2021.135873. Epub 2021 Apr 1. Neurosci Lett. 2021. PMID: 33812934 Review.
-
Neurotoxicity of Metal Mixtures.Adv Neurobiol. 2017;18:227-265. doi: 10.1007/978-3-319-60189-2_12. Adv Neurobiol. 2017. PMID: 28889271 Review.
-
Evaluating Manganese, Zinc, and Copper Metal Toxicity on SH-SY5Y Cells in Establishing an Idiopathic Parkinson's Disease Model.Int J Mol Sci. 2023 Nov 9;24(22):16129. doi: 10.3390/ijms242216129. Int J Mol Sci. 2023. PMID: 38003318 Free PMC article.
-
Metals, oxidative stress and neurodegenerative disorders.Mol Cell Biochem. 2010 Dec;345(1-2):91-104. doi: 10.1007/s11010-010-0563-x. Epub 2010 Aug 22. Mol Cell Biochem. 2010. PMID: 20730621 Review.
Cited by
-
Global trends and projections of Parkinson's disease incidence: a 30-year analysis using GBD 2021 data.J Neurol. 2025 Mar 25;272(4):286. doi: 10.1007/s00415-025-13030-2. J Neurol. 2025. PMID: 40131471
-
Exploring Risk and Protective Factors in Parkinson's Disease.Cells. 2025 May 14;14(10):710. doi: 10.3390/cells14100710. Cells. 2025. PMID: 40422213 Free PMC article. Review.
-
Metabolomic and Lipidomic Analysis of Manganese-Associated Parkinsonism: a Case-Control Study in Brescia, Italy.medRxiv [Preprint]. 2024 Sep 6:2024.09.04.24313002. doi: 10.1101/2024.09.04.24313002. medRxiv. 2024. PMID: 39281765 Free PMC article. Preprint.
-
Multifunctional Metallothioneins as a Target for Neuroprotection in Parkinson's Disease.Antioxidants (Basel). 2023 Apr 6;12(4):894. doi: 10.3390/antiox12040894. Antioxidants (Basel). 2023. PMID: 37107269 Free PMC article. Review.
-
In vitro senescence and senolytic functional assays.Biomater Sci. 2025 Jun 25;13(13):3509-3531. doi: 10.1039/d4bm01684j. Biomater Sci. 2025. PMID: 40375674 Review.
References
-
- Ramesh D., Jeong J.H., Yook S. Development of immunotherapy and nanoparticles-based strategies for the treatment of Parkinson’s disease. J. Pharm. Investig. 2021;51:465–481.
-
- Abdellatif A., Hiba O.E., Gamrani H. Copper poisoning induces neurobehavioral features of Parkinson’s disease in rat: Alters dopaminergic system and locomotor performance. Park. Relat. Disord. 2016;22:e188.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

