Sidestream Smoke Extracts from Harm-Reduction and Conventional Camel Cigarettes Inhibit Osteogenic Differentiation via Oxidative Stress and Differential Activation of intrinsic Apoptotic Pathways
- PMID: 36552682
- PMCID: PMC9774253
- DOI: 10.3390/antiox11122474
Sidestream Smoke Extracts from Harm-Reduction and Conventional Camel Cigarettes Inhibit Osteogenic Differentiation via Oxidative Stress and Differential Activation of intrinsic Apoptotic Pathways
Abstract
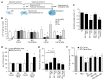
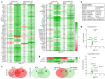
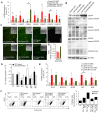
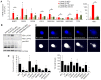
Epidemiological studies suggest cigarette smoking as a probable environmental factor for a variety of congenital anomalies, including low bone mass, increased fracture risk and poor skeletal health. Human and animal in vitro models have confirmed hypomineralization of differentiating cell lines with sidestream smoke being more harmful to developing cells than mainstream smoke. Furthermore, first reports are emerging to suggest a differential impact of conventional versus harm-reduction tobacco products on bone tissue as it develops in the embryo or in vitro. To gather first insight into the molecular mechanism of such differences, we assessed the effect of sidestream smoke solutions from Camel (conventional) and Camel Blue (harm-reduction) cigarettes using a human embryonic stem cell osteogenic differentiation model. Sidestream smoke from the conventional Camel cigarettes concentration-dependently inhibited in vitro calcification triggered by high levels of mitochondrially generated oxidative stress, loss of mitochondrial membrane potential, and reduced ATP production. Camel sidestream smoke also induced DNA damage and caspase 9-dependent apoptosis. Camel Blue-exposed cells, in contrast, invoked only intermediate levels of reactive oxygen species insufficient to activate caspase 3/7. Despite the absence of apoptotic gene activation, damage to the mitochondrial phenotype was still noted concomitant with activation of an anti-inflammatory gene signature and inhibited mineralization. Collectively, the presented findings in differentiating pluripotent stem cells imply that embryos may exhibit low bone mineral density if exposed to environmental smoke during development.
Keywords: developmental toxicity; embryonic stem cells; hypomineralization; osteoblasts; oxidative stress; tobacco smoke solution.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Exposure to Cigarette Smoke Impedes Human Osteoblast Differentiation Independently of Nicotine.Nicotine Tob Res. 2022 Nov 12;24(12):1921-1926. doi: 10.1093/ntr/ntac144. Nicotine Tob Res. 2022. PMID: 35778911
-
Comparison of toxicity of smoke from traditional and harm-reduction cigarettes using mouse embryonic stem cells as a novel model for preimplantation development.Hum Reprod. 2009 Feb;24(2):386-97. doi: 10.1093/humrep/den419. Epub 2008 Nov 29. Hum Reprod. 2009. PMID: 19043081
-
Embryonic Exposure to Cigarette Smoke Extract Impedes Skeletal Development and Evokes Craniofacial Defects in Zebrafish.Int J Mol Sci. 2022 Aug 31;23(17):9904. doi: 10.3390/ijms23179904. Int J Mol Sci. 2022. PMID: 36077301 Free PMC article.
-
Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke.Tob Control. 2005 Dec;14(6):396-404. doi: 10.1136/tc.2005.011288. Tob Control. 2005. PMID: 16319363 Free PMC article. Review.
-
Smoking and reproduction: the oviduct as a target of cigarette smoke.Reprod Biol Endocrinol. 2005 Sep 28;3:52. doi: 10.1186/1477-7827-3-52. Reprod Biol Endocrinol. 2005. PMID: 16191196 Free PMC article. Review.
Cited by
-
Mediating role of inflammatory markers in the relationship between cotinine levels and total bone mineral density.PLoS One. 2025 Aug 8;20(8):e0329062. doi: 10.1371/journal.pone.0329062. eCollection 2025. PLoS One. 2025. PMID: 40779502 Free PMC article.
-
Differences in Airway Remodeling and Emphysematous Lesions between Rats Exposed to Smoke from New-Type and Conventional Tobacco Varieties.Antioxidants (Basel). 2024 Apr 24;13(5):511. doi: 10.3390/antiox13050511. Antioxidants (Basel). 2024. PMID: 38790616 Free PMC article.
-
Perturbations in Osteogenic Cell Fate Following Exposure to Constituents Present in Tobacco: A Combinatorial Study.Toxics. 2023 Dec 7;11(12):998. doi: 10.3390/toxics11120998. Toxics. 2023. PMID: 38133399 Free PMC article.
-
Endocrine Disruptor-Induced Bone Damage Due to Hormone Dysregulation: A Review.Int J Mol Sci. 2023 May 5;24(9):8263. doi: 10.3390/ijms24098263. Int J Mol Sci. 2023. PMID: 37175969 Free PMC article. Review.
-
Therapeutic Potential of Thiophene-Based Chalcone Analog Against Acrylamide-Induced Neurotoxicity and Osteotoxicity.Mol Neurobiol. 2025 May;62(5):5730-5743. doi: 10.1007/s12035-024-04623-5. Epub 2024 Dec 2. Mol Neurobiol. 2025. PMID: 39617840
References
-
- Centers for Disease Control and Prevention (CDC) Smoking prevalence among women of reproductive age—United States, 2006. MMWR Morb. Mortal. Wkly. Rep. 2008;57:849–852. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

