Ellagic Acid Triggers the Necrosis of Differentiated Human Enterocytes Exposed to 3-Nitro-Tyrosine: An MS-Based Proteomic Study
- PMID: 36552693
- PMCID: PMC9774974
- DOI: 10.3390/antiox11122485
Ellagic Acid Triggers the Necrosis of Differentiated Human Enterocytes Exposed to 3-Nitro-Tyrosine: An MS-Based Proteomic Study
Abstract
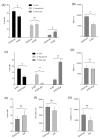
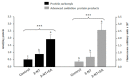
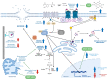
To study the molecular basis of the toxicological effect of a dietary nitrosated amino acid, namely, 3-nitrotyrosine (3-NT), differentiated human enterocytes were exposed to dietary concentrations of this species (200 μM) and analyzed for flow cytometry, protein oxidation markers and MS-based proteomics. The possible protective role of a dietary phytochemical, ellagic acid (EA) (200 μM), was also tested. The results revealed that cell viability was significantly affected by exposure to 3-NT, with a concomitant significant increase in necrosis (p < 0.05). 3-NT affected several biological processes, such as histocompatibility complex class II (MHC class II), and pathways related to type 3 metabotropic glutamate receptors binding. Addition of EA to 3-NT-treated cells stimulated the toxicological effects of the latter by reducing the abundance of proteins involved in mitochondrial conformation. These results emphasize the impact of dietary nitrosated amino acids in intestinal cell physiology and warn about the potential negative effects of ellagic acid when combined with noxious metabolites.
Keywords: 3-Nitro-L-tyrosine; MHC class II; ellagic acid; flow cytometry; mitochondria; protein oxidation; proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Protein oxidation marker, α-amino adipic acid, impairs proteome of differentiated human enterocytes: Underlying toxicological mechanisms.Biochim Biophys Acta Proteins Proteom. 2022 Jul 1;1870(7):140797. doi: 10.1016/j.bbapap.2022.140797. Epub 2022 Jun 9. Biochim Biophys Acta Proteins Proteom. 2022. PMID: 35691541
-
Ellagic acid mitigates arsenic-trioxide-induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells.J Biochem Mol Toxicol. 2018 Feb;32(2). doi: 10.1002/jbt.22024. Epub 2018 Jan 4. J Biochem Mol Toxicol. 2018. PMID: 29314450
-
Serum Metabolomics Based on GC-MS Reveals the Antipyretic Mechanism of Ellagic Acid in a Rat Model.Metabolites. 2022 May 25;12(6):479. doi: 10.3390/metabo12060479. Metabolites. 2022. PMID: 35736412 Free PMC article.
-
Ellagic Acid as a Tool to Limit the Diabetes Burden: Updated Evidence.Antioxidants (Basel). 2020 Dec 3;9(12):1226. doi: 10.3390/antiox9121226. Antioxidants (Basel). 2020. PMID: 33287432 Free PMC article. Review.
-
Ellagic Acid: A Dietary-Derived Phenolic Compound for Drug Discovery in Mild Cognitive Impairment.Front Aging Neurosci. 2022 Jul 4;14:925855. doi: 10.3389/fnagi.2022.925855. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35860668 Free PMC article. Review.
Cited by
-
Spore Oil-Functionalized Selenium Nanoparticles Protect Pancreatic Beta Cells from Palmitic Acid-Induced Apoptosis via Inhibition of Oxidative Stress-Mediated Apoptotic Pathways.Antioxidants (Basel). 2023 Mar 30;12(4):840. doi: 10.3390/antiox12040840. Antioxidants (Basel). 2023. PMID: 37107215 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

